Figure 1. Structure and chemistry of synthetic nicotine.
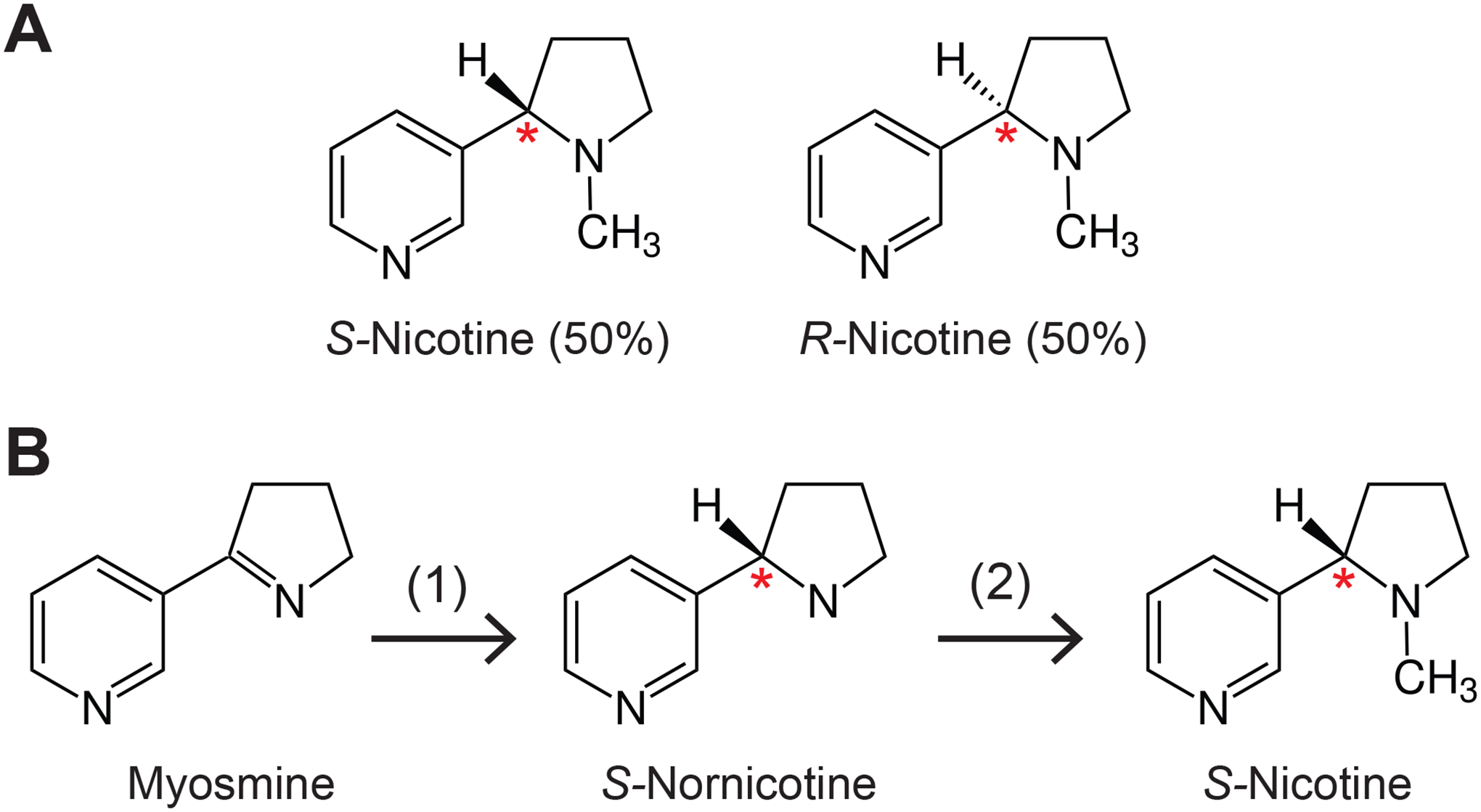
(A). Structures of S- and R-nicotine. The compounds differ in their configuration at the carbon atom labeled with a red asterisk, also called a chiral center. In tobacco leaf >99% of nicotine is present as S-nicotine. Synthetic “Tobacco-Free Nicotine” (TFN), marketed by Next Generation Labs, is racemic, containing 50% S-nicotine and 50% R-nicotine. Pure synthetic S-nicotine is chemically indistinguishable from S-nicotine purified from tobacco.
(B). Synthesis of S-nicotine as described in a patent assigned to Zanoprima involving a biotechnological step. The starting material is myosmine, first converted to S-nornicotine using a recombinant enzyme (1), a NADH/NADPH dependent imine reductase. Such a reaction is also called “stereoselective”. S-nornicotine is then converted to S-nicotine through methylation (2).
