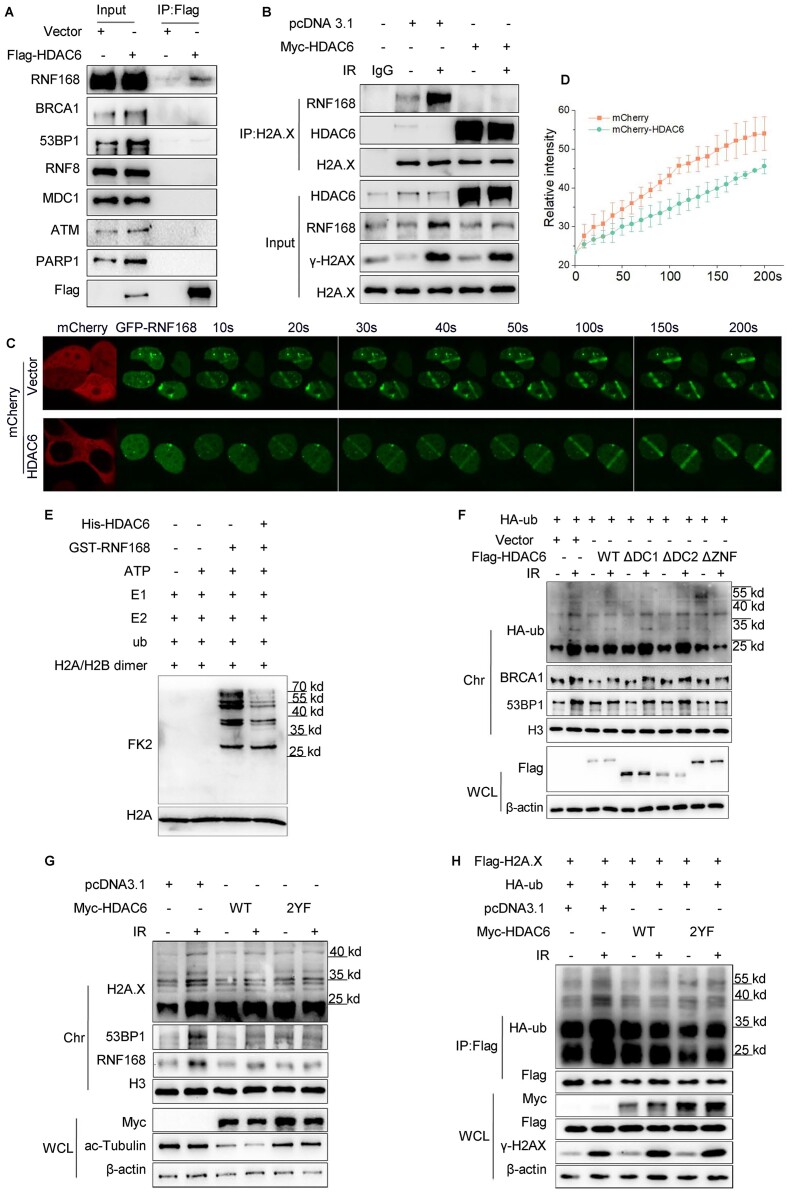
Figure 4.
HDAC6 regulates the H2A/H2A.X ubiquitination signaling cascade in an RNF168-dependent manner during DSB repair. (A) HeLa cells were transfected with Flag-HDAC6 or an empty plasmid for 48 h. Whole cell lysates were subjected to immunoprecipitation with an anti-Flag antibody before western blotting. (B) HeLa cells were transfected with Myc-HDAC6 or empty plasmid for 48 h and exposed to 10 Gy irradiation (IR) and released for 1 h. Chromatin fractions were subjected to immunoprecipitation with an anti-H2A.X antibody followed by western blotting. Rabbit IgG was used as a negative control. (C, D) HeLa cells were co-transfected with GFP-RNF168 and mCherry-HDAC6 or an empty plasmid for 48 h and then subjected to laser micro-IR. Images were captured every 10 s for 200 s (C), and the IR path signal intensity was quantified (D). (E) Recombinant His-HDAC6 and GST-RNF168 were subjected to in vitro ubiquitination assays in the presence of ATP, E1 (UBE1), E2 (UbcH5c), ub (ubiquitin) and H2A/H2B dimer as indicated. Western blotting was performed with the indicated antibodies. (F, G) HeLa cells were transfected with the indicated plasmids for 48 h and then exposed to 10 Gy irradiation and released for 1 h. Whole cell lysate and chromatin fractions were analyzed by western blotting with the indicated antibodies. WT: wild-type; ΔDC1: DAC1 domain deleted; ΔDC2: DAC2 domain deleted; ΔZnF: ZnF-UBP domain deleted (F); HDAC6 catalytic mutant (Y386F/Y782F) (G). (H) HeLa cells were co-transfected with Flag-H2A.X, HA-ub, with Myc-HDAC6 WT or Myc-HDAC6 2YF (catalytic mutant) for 48 h and then exposed to 10 Gy irradiation and released for 1 h. The Flag-H2A.X proteins were immunoprecipitated from the whole cell lysates and eluted to detect the ubiquitination changes by western blotting with the indicated antibodies. All data represent the means ± SD.