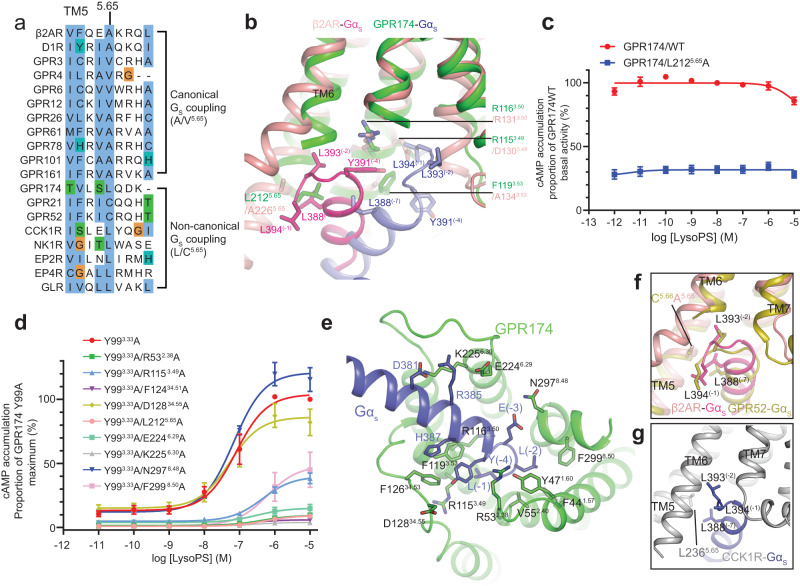
Fig. 6. The non-canonical Gs coupling mode is determined by a larger hydrophobic residue at position 5.65 of TM5.
a Receptors that adopt canonical Gs coupling mode have a small hydrophobic residue A/V at position 5.65, while receptors that adopt non-canonical Gs coupling prefer a large hydrophobic residue (L/C5.65). b Comparison of the binding interface between β2AR–Gαs and GPR174–Gαs. The hook of Gαs is distorted when bound to GPR174 due to a potential steric clash between L2125.65 and the tri-leucine pocket formed by L394(-1), L393(−2), and L388(−7). c Mutation of L2125.65 in GPR174 remarkably reduces its basal activity. Each data point represents mean ± SEM from three independent experiments. Source data are provided as a Source Data file. d The effects of mutations in the GPR174‒Gs interface on the potency and maximum effect of lysoPS in the context of the Y993.33A mutant evaluated by the cAMP assay. Each data point represents mean ± SEM from three independent experiments. Source data are provided as a Source Data file. e Detailed interactions between GPR174 and Gαs. f and g The presence of a larger hydrophobic residue (L or C) at 5.65 in GPR52 (f) and CCK1R (g) leads to a non-canonical Gs coupling mode.