Figure 1. Overview of the structure and the key components of the Gram-negative cell envelope.
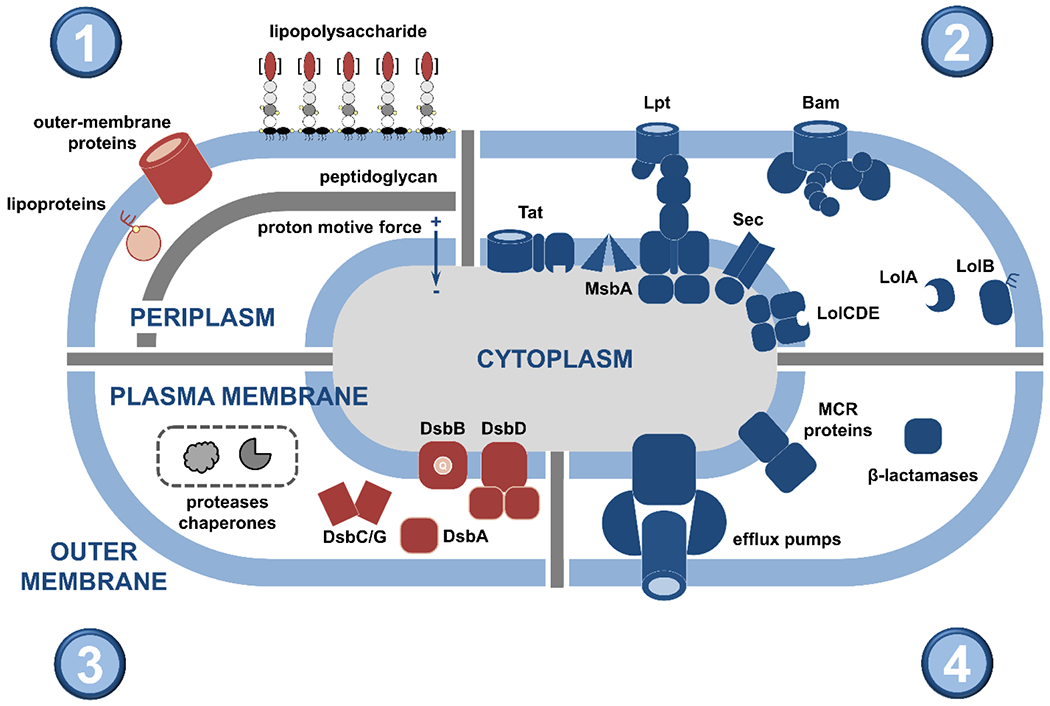
This review article discusses the potential of the constituents and processes depicted here as targets for the generation of novel strategies against bacterial pathogens. (1; top left) The cell envelope is composed of the outer membrane, the peptidoglycan layer, and the plasma membrane. The asymmetric outer membrane is composed of glycolipid lipopolysaccharide and phospholipids that are located in its extracellular and periplasmic leaflets, respectively. Embedded within this lipidic environment, β-barrel proteins aid nutrient transport and act as receptors, and lipoproteins perform a range of functions often linked to the biogenesis of the cell envelope. The rigid peptidoglycan layer is attached to the outer membrane, helping the cell retain its shape against a variety of stresses. Separating the periplasm from the cytoplasm, the phospholipid-rich plasma membrane is a platform for numerous cellular processes that connect the cell interior with the extracellular environment, many of which are energized by the proton motive force. (2; top right) Cell envelope biogenesis and function rely on the translocation of newly synthesized proteins across the plasma membrane by two translocation systems, the general secretory pathway (Sec) and the twin arginine pathway (Tat). Their substrates are inserted into the plasma membrane, released into the periplasm, extruded to the extra-cellular space, or passed on to other protein partners for further processing. The latter includes the Lol pathway, which is involved in the biogenesis of lipoproteins, and the Bam pathway that is responsible for the localization and folding of outer-membrane β-barrels. Generation and transport of the lipopolysaccharide occurs separately and relies on specialized cytoplasmic machinery, the flippase MsbA (which moves the lipopolysaccharide precursor to the periplasmic leaflet of the plasma membrane) and the Lpt pathway. (3; bottom left) Cell envelope biogenesis, maintenance, and homeostasis rely on complex quality control networks. Major players include soluble chaperones, involved in shuttling proteins and lipids across the periplasm to their final destinations, proteases that degrade misfolded and aggregated proteins, and members of the disulfide bond formation pathway (DsbA-D shown here) that perform oxidative protein folding. (4; bottom right) Many broad-acting antimicrobial resistance determinants are located in the cell envelope, such as β-lactamase enzymes that break down antibiotics like penicillin, mobile colistin resistance (MCR) proteins, which protect the cell against last-resort polymyxin drugs, and efflux pumps, responsible for extruding many different classes of antibiotics.
