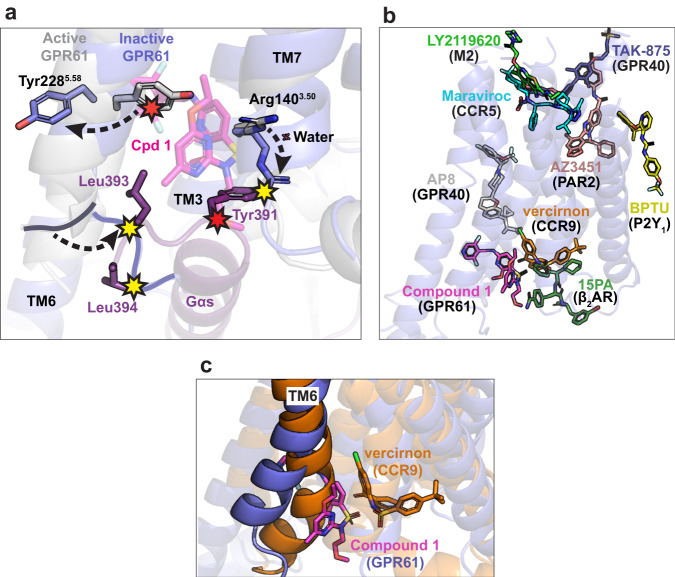
Fig. 4. Analysis of compound 1 inverse agonist mechanism.
a Key residue clashes and conformational changes induced by binding of compound 1 (Cpd 1) to GPR61. The structure of active GPR61 (light grey) is overlaid with the compound 1 (magenta)-bound structure of inactive GPR61 (blue), with key residues highlighted in stick representation. Clashes with the compound are indicated by red stars, while clashes with Gαs induced by compound binding are indicated by yellow stars. b Compound 1 defines an unusual allosteric site and mechanism. The structure of compound 1-bound GPR61IA is shown in ribbon representation, with published exemplars45,62–68 representing the known allosteric sites of class A GPCRs superimposed, colored as indicated. c Compound 1-bound GPR61IA and vercirnon-bound CCR945 structures, colored as indicated, are superimposed. Vercirnon occupies the known allosteric site that lies nearest to that of compound 1. The different conformations of TM6 induced by these two inverse agonists are highlighted.