Fig. 5. A G protein-competitive mechanism of allosteric GPCR inverse agonism.
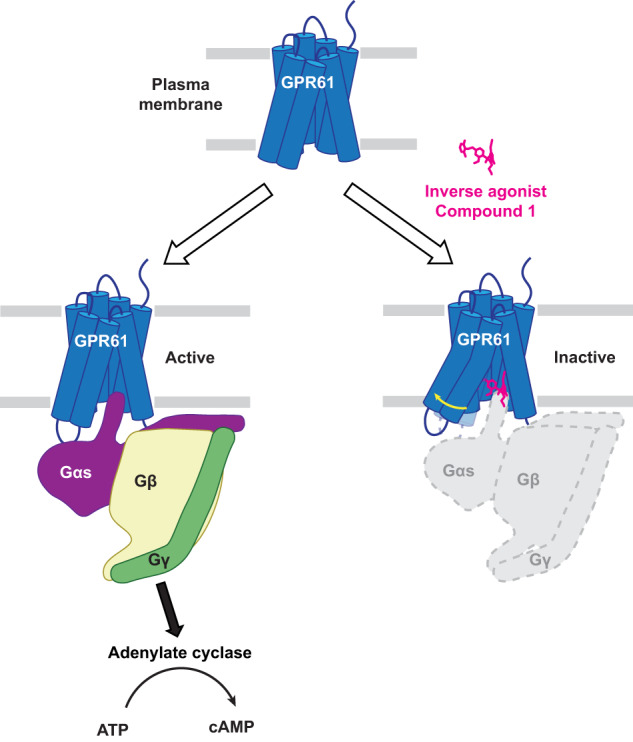
In its constitutively active state (top panel), GPR61 adopts a conformation that allows binding and nucleotide exchange of the G protein complex (bottom left panel) to stimulate cyclic AMP-mediated signaling through activation of adenylate cyclase. Inverse agonist compound 1 (magenta) binds to an intracellular allosteric pocket overlapping that bound by Gαs and acts as a wedge to remodel the flanking helices (yellow arrow), destroying the Gαs-binding pocket and creating direct clashes that prevent potential Gαs binding.
