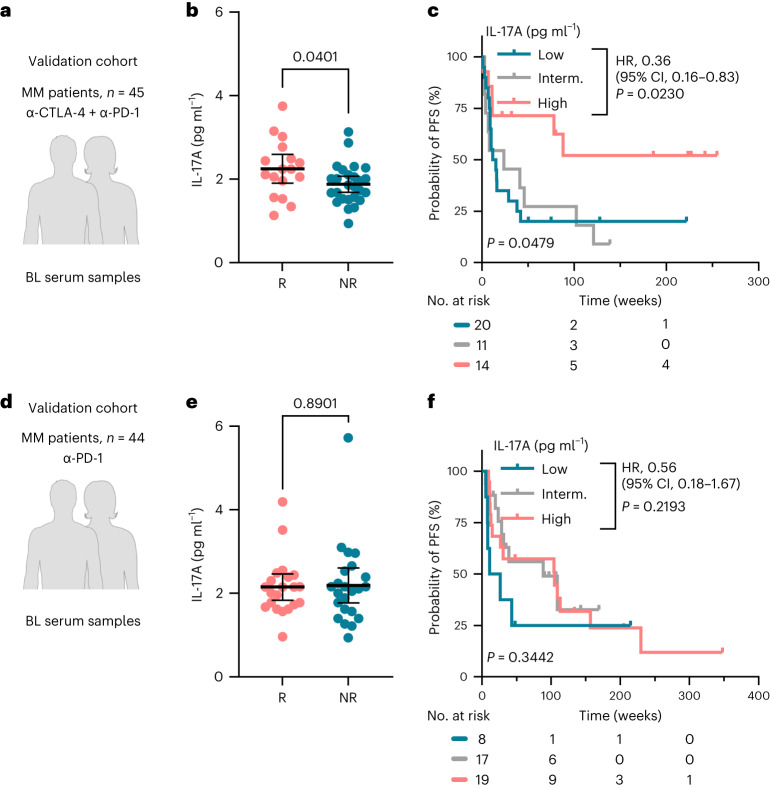
Fig. 6. Validation cohort.
a, Dual-ICI-treated melanoma validation cohort (anti-CTLA-4 and anti-PD-1 antibodies, n = 45). b, Serum IL-17A levels as measured by ELISA in correlation to the best clinical response (n = 17 responders versus n = 26 non-responders) in samples collected at therapy baseline. c, Kaplan–Meier plot for PFS according to the baseline IL-17A concentration. d, Mono anti-PD-1-treated melanoma validation cohort (anti-PD-1 therapy, n = 44). e, Serum IL-17A levels as measured by ELISA in correlation to the best clinical response (n = 21 responders versus n = 23 non-responders) in samples collected at therapy baseline. f, Kaplan–Meier plot for PFS according to the baseline IL-17A concentration. P values are from the unpaired t-test, and mean ± 95% CIs are plotted in b,e. Each dot represents a biologically independent sample. Categorization into ‘high’ versus ‘low’ according to the X-tile-determined cut-point value was carried out separately within each dataset. HRs and 95% CIs are reported for ‘IL-17A high’, and P values are from the log-rank test in c,f. All P values are two tailed. Responder, complete and partial response; non-responder, progressive disease, stable disease, mixed response.