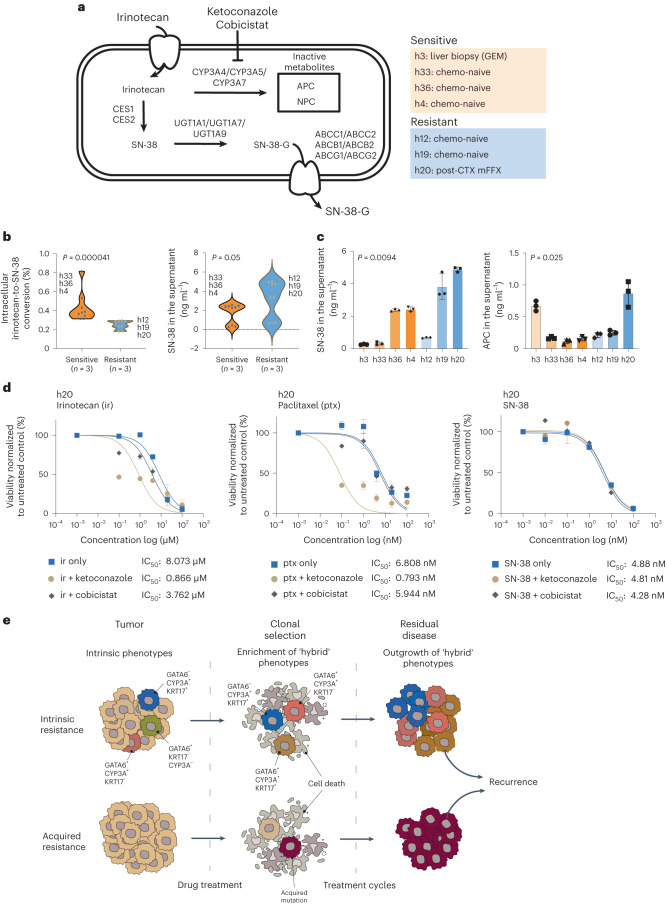
Fig. 8. CYP3A activity mediates irinotecan tolerance in CYP3A+ PDOs.
a, Irinotecan is converted to the active metabolite SN-38 in liver and small intestinal epithelial cells and also pancreatic cancer cells. CYP3A proteins may metabolize irinotecan into inactive metabolites APC and NPC, leading to drug tolerance. SN-38 may also undergo glucuronidation and be exported from cancer cells. CYP3A inhibitors, such as ketoconazole and cobicistat, may overcome irinotecan drug tolerance by increasing the accumulation of SN-38. b, Compound analysis by UPLC–MS/MS of irinotecan metabolites in relative resistant (n = 3) and relative susceptible (n = 3) PDOs showing intracellular irinotecan-to-SN-38 conversion (left) and SN-38 accumulation in the supernatant (right). Biological replicates (n = 3) for the representative PDOs are shown in the plots. Wilcoxon rank-sum test two-sided P values are shown on the plots. P values were not adjusted for multiple testing. c, Compound analysis by UPLC–MS/MS of relative resistant and relative susceptible PDOs showing the accumulation of SN-38 or inactive metabolite APC in the supernatant following irinotecan treatment. The results represent n = 3 independent biological experiments. Data are presented as mean ± s.d. One-way analysis of variance (two-tailed) P values are shown on the plots. P values were not adjusted for multiple testing. d, Treatment of an irinotecan-resistant PDO (h20) with irinotecan, paclitaxel and SN-38 in combination with either ketoconazole or cobicistat as indicated. Combination treatment with ketoconazole increases drug sensitivity to irinotecan and paclitaxel but not SN-38. The results represent n = 3 independent biological experiments. Data are presented as mean ± s.e.m. IC50 values are provided for the indicated treatments. e, Alternative models to explain drug tolerance and persistence in post-CTX samples. Intrinsic resistance: treatment-mediated selection of preexisting drug-tolerant phenotypes may shape residual disease. This process may involve non-genetic mechanisms, including intrinsic and/or drug-induced expression of drug-detoxifying genes and/or a transition toward basal-like or ‘hybrid’ states from predominant classical states due to intrinsic neoplastic plasticity. Acquired resistance: rare subclones acquire a drug-resistant driver alteration before or during therapy. These resistant clones expand and eventually drive relapse due the clonal acquisition of the preexisting drug-resistant mechanism.