Figure 1.
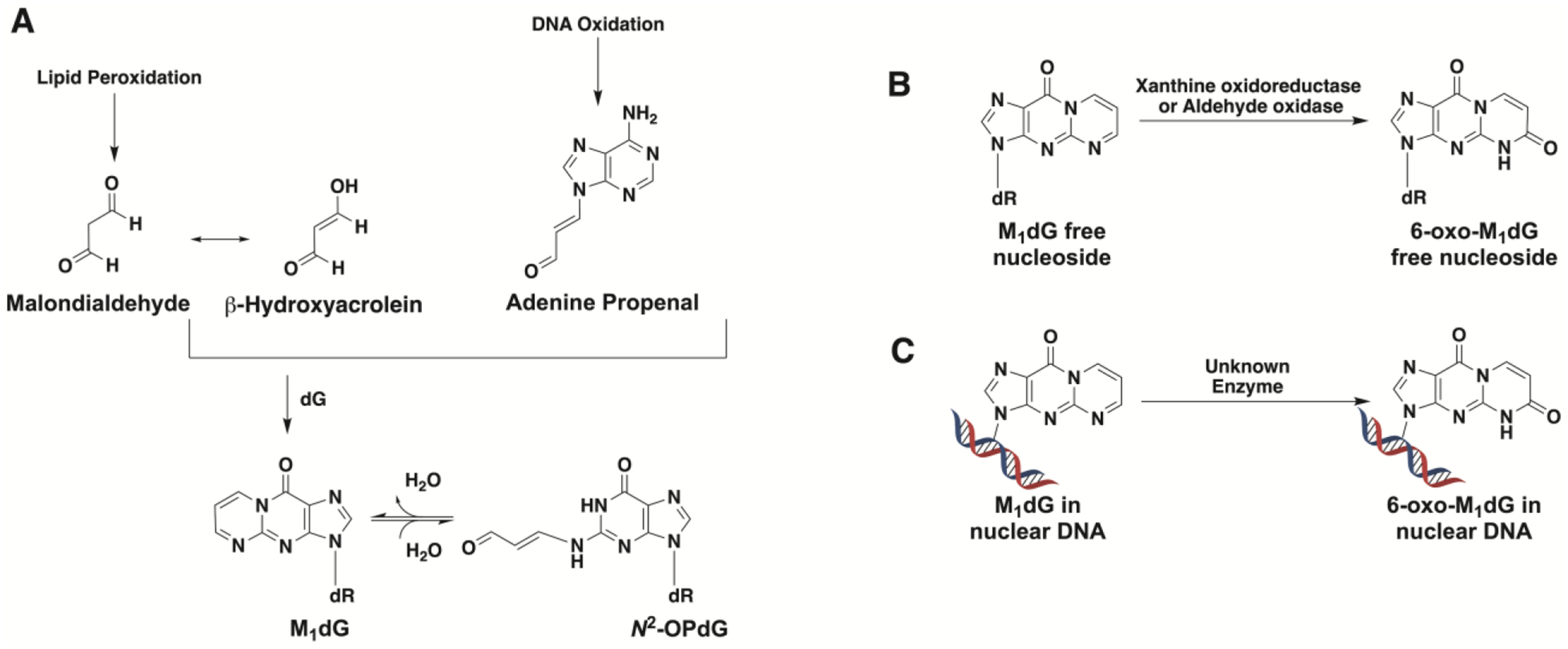
(A) Formation of M1dG from the lipid peroxidation product malondialdehyde via its tautomer β-hydroxyacrolein or from the DNA oxidation product adenine propenal. M1dG is subject to ring-opening to form N2-(3-oxo-1-propenyl)-dG (N2-OP-dG). (B) M1dG generated by base excision repair of damaged DNA is oxidized to 6-oxo-M1dG by xanthine oxidoreductase or aldehyde oxidase. (C) M1dG in nuclear DNA is oxidized to 6-oxo-M1dG.
