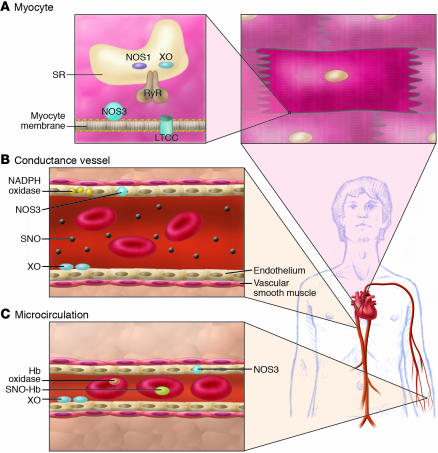
Figure 3.
Consequences of NO/redox disequilibrium in the cardiovascular system – congestive HF phenotype. The balance between nitric oxide (NOS and hemoglobin–based activities) and superoxide/ROS production (oxidase activity) plays a pivotal role in cell/organ function at key sites in the cardiovascular system, including the heart (A), large- and medium-sized conductance blood vessels (B), and the microvasculature (C) (S12). At each of these sites, NO/redox disequilibrium is identified with dysregulated NO-based signaling. (A) In the cardiac myocyte, NO regulates receptor-mediated signal transduction, the calcium cycle, mitrochondrial respiration, and myofilament contractility. Loss of NOS in the cardiac SR (95) impairs NO signaling and creates oxidative stress (by relieving inhibition of the oxidase) (22). Upregulation of inducible NOS (NOS2) may further disrupt physiologic NO regulation by producing a nitrosative stress. The NO/redox disequilibrium that ensues in HF is characterized by disruption and/or impairment of the cardiac calcium cycle, mitochondrial respiration, and myofilament responsiveness to activator calcium. (B) In conductance vessels, vasoconstriction may result from diminished endothelial NOS activity and/or impaired delivery of plasma-borne NO bioactivity. A NO/redox disequilibrium is linked to increased expression or activity of both vascular NADPH oxidase (Nox4) (99) and circulating XO (46). (C) In the microvasculature, rbcs govern NO bioactivity. Lower venous O2 saturation in HF may subserve a NO/redox disequilibrium by impairing NO release from rbcs (SNO-Hb) and promoting hemoglobin oxidase activity (44, 75). Impaired vasodilation by rbcs may exacerbate tissue ischemia.