Abstract
Acute pancreatitis, a potentially fatal disease, with symptoms including nausea and/or vomiting, indigestion, and abdominal pain, is known to range from a mild self-limiting state up to a more severe and lethal form. This review aims to provide a clearer picture to improve understanding the role of viral agents in the development of acute pancreatitis. Common databases including PubMed, Google Scholar, and Scopus were used for the literature search. In this review search terms including virus, viral, infection, and specific descriptive terms for a virus were considered in different combinations. Various causative agents are recognized in the development of acute pancreatitis as one of the most frequent gastrointestinal diseases, such as gallstones, alcoholism, and hypertriglyceridemia. Microbial pathogens with about 10% of acute pancreatitis cases, mainly viruses, among other factors, are thought to play a role in this regard. Once the pancreatitis diagnosis has been made, depending on the causative agent, the management approach and specific interventions affect the final outcome. Virus-induced acute pancreatitis in patients should be considered. Advanced diagnostic tests such as PCR, in situ hybridization, and biopsy can help for a better understanding of the role of viruses in causing acute pancreatitis. Improvement in the tests will lead to timely diagnosis, treatment, and better management of pancreatitis.
Key Words: Acute pancreatitis, Virus, Gastrointestinal, Abdominal pain
Introduction
Acute pancreatitis is one of the most frequent gastrointestinal diseases for admission to hospital, whose annual incidence has been reported at 34 per 100,000 person-years in developed countries, with no significant relationship with gender (1). Acute pancreatitis is caused by a series of inflammatory processes within the pancreas and is usually associated with abdominal pain, vomiting, and nausea. The clinical course of the disease is variable depending on the inflammatory response level, local or systemic. Mild acute pancreatitis, with a self-limiting course, is more common in patients and recovery is achieved within one week. Approximately 20 to 30% of patients indicate the moderate or severe acute pancreatitis form, a life-threatening condition. Although the overall mortality rate in acute pancreatitis is low and occurs in 1% of cases, the hospital mortality rate in the severe form is about 15% (2, 3).
Meanwhile, exposure to various risk factors and diagnostic practices can play an important role in these results. The main causes of acute pancreatitis in high-income countries are gallstones (45%), alcohol use (20%), and hypertriglyceridemia (10%). Additional causes such as infection, genetics, endoscopic retrograde cholangiopancreatography (ERCP), autoimmune diseases, hypercalcemia, and medication have also been reported (4).
According to the Atlanta Criteria, the diagnosis of acute pancreatitis is possible based on two of the following three criteria: upper abdominal pain, an elevated serum lipase or amylase level (or both) greater than three times the upper normal threshold, and abdominal imaging findings (3). Acute pancreatitis can progress to recurrent acute pancreatitis and eventually lead to chronic pancreatitis. Acute pancreatitis can relapse in 20% to 30% of people and in 10% of people, it can develop into chronic pancreatitis (5).
Since chronic pancreatitis is considered a factor in the development of pancreatic cancer, the role of pancreatitis-causing factors seems to be important in this cancer. Infectious agents such as viruses are among these causes (6). Also, since pancreatitis affects the patient’s quality of life through abdominal pain, vomiting, exocrine and/or endocrine dysfunction, jaundice, and weight loss, understanding the etiologies, details of pathological mechanisms and influential factors of this disease is very crucial. About 10% of pancreatitis is thought to be caused by infectious agents, mainly viral, through direct viral injury or the immune-mediated inflammatory response to viral infection (7). The possible molecular mechanisms of viruses in pancreatic complications have not been elucidated. Nevertheless, inflammations and continuous immune responses to viral proteins, molecular mimicry, virus-host proteins interactions might be involved in the process. This research aims to explore the possible roles of viral agents in the development of pancreatitis through literature review.
Search Strategy
Common databases including PubMed, Google Scholar, and Scopus were used for the literature search. In this review search terms including virus, viral, infection, and specific descriptive terms for a virus such as “human immunodeficiency virus” or “HIV” with pancreatitis, pancreas, and pancreatic were considered in different combinations. The inclusion criteria were as follows: original articles, case reports, and case series. The exclusion criteria included reviews, duplicates, and articles whose full text was not available or were not in the English language. The search was limited to human studies published up to September 2021.
Pancreatitis: pathophysiology and etiology
Acute pancreatitis, one of the most common gastrointestinal diseases, is the result of a rapid inflammatory response to pancreatic damage. Injured pancreatic acinar cells trigger the activation of trypsinogen to trypsin and the initial neutrophils’ recruitment into the pancreas. In turn, activated trypsin converts the other pancreatic digestive pro-enzymes to active enzymes. Ultimately, these enzymes result in the process of self-digestion of the pancreatic cells and the destruction of the pancreatic tissue. In most patients, this positive feedback loop usually stops spontaneously. However, in some persons, the disease progresses and causes a very serious illness, leading to widespread pancreatic necrosis (3).
Gallstones are the most common cause of acute pancreatitis in most high-income countries, accounting for approximately 40% of cases. Pancreatic duct obstruction caused by gallstones, raises pressure within the pancreatic duct, which causes the bile acid to return back into the pancreas and the activation of trypsinogen inside the pancreas, can lead to tissue injury (8). Excessive alcohol consumption causes 25% of acute pancreatitis cases around the world and is the second most common etiology of acute pancreatitis. Most commonly, alcoholic pancreatitis occurs in males, with the mean age of almost 40 years, with a range of alcohol consumption around 100 to 150 g/day equal to ~ 4-5 drinks daily) for over five years (9).
Despite the long-standing recognition of this association, the mechanisms by which ethanol influences acute pancreatitis development are poorly understood where both genetic and non-genetic associated factors can contribute to the progression of this disease. Ethanol through its direct toxic effects and metabolites sensitizes acinar cells to cholecystokinin, which leads to increased trypsin production in the pancreas and reduced threshold for the development of pancreatitis (3).
Hypertriglyceridemia, the third most common etiology of pancreatitis, accounts for approximately 10% of all cases of pancreatitis in the world. A serum triglyceride level greater than 1000 mg/dL typically increases the risk of acute pancreatitis. Pancreatic lipase breaks down triglycerides into toxic free fatty acids (FFA) and leads to induction of inflammatory changes and tissue damage in the pancreas (10). Rare causes of acute pancreatitis include medications, autoimmune etiologies, genetic factors, toxins, and infectious microorganisms.
Viruses associated with acute pancreatitis
Among the infectious agents, viruses including SARS-CoV-2, hepatitis viruses, EBV, CMV, HSV, varicella-zoster virus, Coxsackie virus, mumps, measles, HIV, and other viruses play the most important role in the development of acute pancreatitis (Table 1). Tropism and the replication of viruses in pancreas tissue such as SARS-CoV-2 can infect the tissue through ACE2 and TMPRSS2 receptors, which are expressed on exocrine and endocrine cells in human pancreatic tissue (11). Some evidence suggests tropism of the IAV for the pancreas. The expression of human-like (α-2,6-linked) sialic acid (SA) receptor by the human pancreatic cancer cell line PANC-1 and the replication of IAV subtypes in vitro have been reported in a study (12). Additionally, persistent infections by coxsackieviruses-B (CV-B) have been previously established in vitro as well as in vivo (13). Nevertheless, in addition to direct viral infection in pancreas tissue, other indirect mechanisms have been mentioned in the development of virus-induced acute pancreatitis.
Table 1.
Overall results of cases of acute pancreatitis associated with viral infections in case report and case-series studies
| Number; Male/female |
Median age in years (range) | Method of diagnosis of infection | Therapy | Ref | |
|---|---|---|---|---|---|
| HBV | 6/2 | 44 (27-64) | PCR for HBV DNA | NR | (15, 16, 81, 84) |
| HCV | 8/4 | 50 (38-65) | PCR for HCV RNA | Stopping use of Peginterferon | (17-19, 85) |
| HAV | 27/14 13 NR |
16 (2-81) | anti-HAV IgM & RNA | Conservative surgery, and hemodialysis |
(86) |
| HEV | 36/2 18 NR |
28 (7-54) | anti-HEV IgM & RNA | Conservative, surgery, and hemodialysis |
(82, 87) |
| SARS-CoV-2 | 28/34 | 42 (7-76) | PCR for SARS-CoV-2 RNA | NR | (30) |
| VZV | 5/6 | 44 (2-86) | IgM antibodies & VZV DNA | Acyclovir | (32-39, 88-91) |
| CMV | 6/3 | 45 (21-75) | IgM antibodies & CMV DNA | Ganciclovir | (40-44, 92, 93) |
| EBV | 6/10 | 16 (3–39) | IgM antibody | Acyclovir & supportive | (48, 94, 95) |
| HSV | 3/2 | 24 (21-73) | Cowdry type A Inclusions, IgM & DNA HSV |
NR | (49-51, 96, 97) |
| HIV | 6/2 | 37 (17-52) | p24 HIV-1 antigen and PCR for HIV RNA | Stopping use of antiretroviral | (98-104) |
| INFLUENZA | 2/1 | 37 (19-42) | PCR for influenza RNA | Oseltamivir | (61-63) |
| COXSACKIE VIRUS | 2/2 19 NR |
56 (39-67) | IgM titer against CV A4, B1, B2, B4 and B5 | NR | (64, 65, 105-107) |
| ROTAVIRUS | 4/1 | 2 (1-10) | Stool examination with culture and Immuno-chromatography kit | Intravenous rehydration | (67) |
| MUMPS | 2/3 | 46 (19-72) | IgM antibody | Symptomatic and supportive |
(73, 75, 108, 109) |
| MEASLES | 2/2 | 16 (2-22) | IgM antibody | Symptomatic and supportive |
(76-79) |
NR: not reported.
Hepatitis B virus (HBV)
HBV is one of the viruses infecting the pancreas. Several studies have shown the role of acute hepatitis B virus infection in the development of acute pancreatitis. In a study by Jain et al. on 54 patients with acute hepatitis B virus infection, after four weeks of follow-up, one (2%) of them showed signs of acute pancreatitis (14). Meanwhile, immunosuppressive therapy after organ transplantation in people with chronic HBV infection can result in acute pancreatitis. On the contrary, Ohshiro et al. reported acute pancreatitis in a patient with acute/non-fulminant HBV infection after reducing the dose of an immunosuppressive agent (15). Yuen et al. reported that 5.6% of acute pancreatitis cases (5 patients) in the Chinese were possibly induced by chronic HBV infection. The mortality rate in the HBV group (concomitantly chronic HBV infection and acute pancreatitis) was higher (4 out of 5, 80%) compared to the two control groups (85 non-HBV patients with acute pancreatitis and 406 patients with chronic HBV without acute pancreatitis), individuals with pancreatitis (13 out of 85, 15.3%), and those with only acute HBV exacerbation (9 out of 406, 2.2%) (16). So far, several case reports of acute pancreatitis in people with acute hepatitis have been published. In most cases, the development of pancreatitis has been associated with immunosuppressive therapy in transplant recipients with hepatitis B surface antigen (HBsAg) seropositivity.
Hepatitis C virus (HCV)
The first case of acute pancreatitis associated with HCV has been reported in a 70-year-old female with symptoms of abdominal pain, hyperamylasemia, and hyperlipasemia. Severe acute hepatitis was not observed, which may explain pancreatitis is the result of local function by HCV (17). Rarely, an association has been observed between the use of peginterferon and acute pancreatitis (18). Also, in a study, out of 1706 HCV-infected individuals who were treated with IFN alpha-2b and ribavirin (RBV), diagnosis of acute pancreatitis was confirmed in seven patients (0.4%). Pancreatitis was resolved in all seven patients after discontinuation of antiviral therapy, indicating a possible role of this therapy in the development of pancreatitis in patients with chronic HCV (19).
Hepatitis E virus (HEV)
The first documented case of acute pancreatitis related to acute hepatitis E (AHE) was reported by Mishra et al. in 1999 (20). Over the past few years, many case reports have also been published in this regard and are available in the literature. To date, the frequency of acute pancreatitis associated with AHE has estimated in two prospective cohort studies. Raj et al. reported 16 (2.1%) positive of acute hepatitis E cases of the seven hundred ninety patients with acute pancreatitis, with no other causes of pancreatitis (21). Bhagat et al. evaluated three hundred and thirty-four patients with acute pancreatitis admitted from 2004 to 2006 and reported 4 HEV-positive patients (22). Also, two other studies have investigated acute pancreatitis rate in acute hepatitis E patients including four cases of acute pancreatitis among 54 AHE patients by Jain et al, which was performed over a 2-year follow-up period (14); also within a 5-year prospective study, Sudhamshu et al. indicated 18 cases of acute pancreatitis among 286 AHE patients (6.2%) (23).
Hepatitis A virus (HAV)
There is a causal relationship between HAV and acute pancreatitis. To our knowledge, most cases of HAV-related acute pancreatitis have been published in the form of case reports, including four separate reports of the existence of an association between acute hepatitis A virus (HAV) infection and acute pancreatitis in patients. HAV infection was confirmed by the detection of IgM antibodies against HAV (24-26). Epidemiological data on the frequency of pancreatitis developing in HAV infection have been indicated in two articles. Sixteen patients with a positive test for IgM anti-HAV were followed up by Jain P et al., for acute viral hepatitis over three years. None of the patients had a history of pancreatitis signs before the infection. Pancreatitis was positive in two patients and finally, recovery was achieved through conservative management (14). Also, a cross-sectional study by Bhagat et al. reported three confirmed cases of hepatitis A virus-related acute viral hepatitis in 334 patients of acute pancreatitis (22).
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)
Angiotensin-converting enzyme 2 (ACE-2), a receptor for SARS-CoV-2 viral entry, is highly expressed in the pancreatic gland and gastrointestinal epithelial cells. Studies have shown that gastrointestinal symptoms, including diarrhea, vomiting, nausea, and abdominal pain are present in up to 50% of COVID-19 cases (27). Brikman et al. reported a 61-year-old man with SARS-CoV-2 who had two of the three criteria for acute pancreatitis, with no lipase level elevation (28). In another publication, Madhurantakam et al. reported two cases of SARS-CoV-2 infection in patients with acute pancreatitis (29). In a case report, Mazrouei et al. concluded that SARS-CoV-2 infection probably plays a role in acute pancreatitis. A few case series and multiple case reports (21 studies) of SARS-CoV-2 associated with acute pancreatitis were reported in 2020 (30). Inamdar et al. during the COVID-19 pandemic, in a cohort study of patients admitted to 12 hospitals in New York, reported 32 COVID-19 positive from 189 acute pancreatitis patients (17%). They suggested the possibility of a causative role of SARS-CoV-2 in association with acute pancreatitis (31).
Varicella-zoster virus (VZV)
The VZV infection occurs in early childhood and is usually a benign and self-limited illness in immunocompetent individuals. Its complications include moderate fever, headache, myalgia, malaise, and a typical skin rash with different developing stages. Other symptoms include pneumonia, hepatitis, encephalitis, myelitis, retinitis, and acute pancreatitis, which are more common in immunocompromised people. Acute pancreatitis is considered as one of the rare complications of VZV. VZV-induced pancreatitis has mostly been reported in children and immunocompromised adults (32-35). However, multiple cases of VZV-induced pancreatitis have been observed in adults with an immunocompetent system (36-39).
Human cytomegalovirus (HCMV)
Our search in the literature resulted in five case reports of pancreatitis associated with cytomegalovirus in immunocompetent individuals. Chan et al. described a case report of hepatitis and pancreatitis for HCMV in an immunocompetent individual. Liver histology and endoscopic ultrasound did not show other etiologies. After ganciclovir treatment, the viremia was cleared and recovery was achieved (40). Oku et al. reported a 55-year-old man with acute pancreatitis who had a high titer of CMV IgG antibody and without HCMV IgM, indicating reactivation of cytomegalovirus disease (41). In the study of Saeed et al., the patient was a 75-year-old woman with HCMV pancreatitis and gastric perforations. Clinical recovery was observed after the initiation of intravenous ganciclovir (42). Gastrointestinal complications including pancreatitis in immunocompromised patients are much more severe than in immunocompetent people. In a case report by Huayna et al., on two HIV-positive people, secondary pancreatitis associated with HCMV infection was observed. The patients were improved through treatment with ganciclovir (43). In other cases, HCMV pancreatitis has also been reported in AIDS patients who improved with ganciclovir (44, 45).
Epstein-Barr virus (EBV)
The first case of Epstein-Barr virus-associated acute pancreatitis was reported in 1966 (46), with 16 cases documented in the literature from then on, which is more common in young people, 8-35 years old. Although acute pancreatitis involved with acute hepatitis is observed commonly in hepatitis infections such as HAV, HBV, or HEV, in acute hepatitis concurrent with acute pancreatitis, the differential diagnosis of EBV needs to be considered. As an example, Kang et al. reported an 11-year-old female with cholestatic hepatitis and infected with EBV, who had symptoms of abdominal pain as well as vomiting and confirmed pancreatitis. Reactivated EBV infection was diagnosed by viral capsid antigen (VCA) IgM, IgG, and ultimately, recovery was reached with conservative management (47). Acute pancreatitis associated with Epstein-Barr virus infection is usually mild and recovers with conservative management (48).
Herpes simplex virus (HSV)
The results of our survey of the literature indicated five cases of herpes simplex acute pancreatitis. Shintaku et al., reported two acute pancreatitis patients, a 59-year-old woman and a 73-year-old man, with HSV-1 infection which was confirmed by the polymerase chain reaction (PCR) method in autopsy specimens. Also, intranuclear inclusions of Cowdry type A and the ground-glass opacity were observed in the nuclei of infected cells (49). Rand et al. isolated HSV-1 from the gastric contents of a young man with acute pancreatitis, with no evidence of primary infection (50). In the last case, a 13-year-old boy was reported with abdominal pain, vomiting, and an elevation in serum amylase and lipase. IgM antibody for HSV was positive and the patient improved after conservative treatment (51).
Human immunodeficiency virus (HIV)
Although the pancreatitis incidence is not very noticeable, 17 to 30 cases per 100 000 populations, the rate observed is higher in people with HIV. In this regard, risk factors such as nucleoside reverse transcriptase inhibitors (NRTIs) and protease inhibitors (PIs) consumption side effects, and CD4 cell counts have been observed in HIV-positive people receiving antiretroviral therapy (52). Several studies have shown that the use of NRTIs is associated with a high incidence of pancreatitis. It appears that treatment with didanosine, stavudine and or together is associated with a higher rate of pancreatitis (53-57). However, other studies have not found evidence of a link between didanosine or stavudine and the incidence of pancreatitis (58). Studies have shown that PI therapy in people infected with HIV can be associated with hypertriglyceridemia. Although this association has been observed, no significant increase appears to have occurred in the prevalence of hyperlipidemic pancreatitis in HIV-infected patients during those years following the use of PIs (59). Also, in studies conducted on HIV/AIDS individuals with low levels of CD4 cell counts (i.e., CD4<50 cells/mm3), a higher risk of developing acute pancreatitis has been reported (52, 60).
Influenza
The first case of acute pancreatitis caused by influenza has been reported by Blum et al., a 37-year-old man with upper abdominal pain and high levels of amylase. The patient improved with combined antibiotic treatment and Tamiflu (61). In a few other case reports, a possible association of acute pancreatitis with H1N1 influenza has been described. In these patients, the symptoms including abdominal pain, vomiting, and elevated serum amylase or lipase resolved after oseltamivir (Tamiflu) treatment and influenza clearance, further supporting an association (62, 63).
Coxsackievirus
In several case reports, patients with myocarditis and pancreatitis associated with coxsackievirus infection have been observed by serotypes A4, B1, B2, B4, and B5 A (64). The serological findings of 118 patients with acute and chronic pancreatitis show that the Coxsackie-B virus, in addition to developing acute pancreatitis, can also cause relapse chronic pancreatitis in people (65). An interesting finding in the experimental study by Tracy S et al. revealed that pancreatitis and myocarditis can be induced by group B coxsackievirus in mice model (66).
Rotavirus
To date, several case reports have been published regarding acute pancreatitis and rotavirus. The literature search identified five case report publications, on children between the ages of 1 to 10 years (four boys and one girl). Abdominal pain was reported in two cases (67, 68). In three cases, abdominal imaging such as computed tomography (CT) scan showed a mildly enlarged edematous pancreas (69-71). By evaluating these studies, this does not infer that acute pancreatitis associated with rotavirus infection results in severe disease in patients (67).
Mumps
About 4% of mumps infections are linked to pancreatitis (72). The most common virus associated with acute pancreatitis in adults is the mumps virus (73). Also, according to the literature, case reports of mumps pancreatitis without parotitis have been described both in children and adults, although this form of pancreatitis being usually milder (74). Meanwhile, it can even occur in people who are vaccinated against mumps. Nevertheless, it does not seem to be very important and significant, as the incidence of mumps-induced pancreatitis has decreased after the mumps vaccine was introduced (75). Tagajdid et al. reported acute pancreatitis in a 49-year-old woman, with epigastric pain, vomiting, and fever. The IgG and IgM antibodies were positive for mumps, confirming an acute viral infection, and the serological tests were rejected for HIV, EBV, CMV, HBV, and HCV. Infection with mumps can be possible with regard to the patient's age, as vaccine-induced immune response drops over time (73).
Measles virus (MV)
Up to now, several case reports of acute measles-induced pancreatitis have been published. The symptoms of pancreatitis disappeared along with the clearance of the measles virus, indicating the etiological relationship between the measles virus and pancreatitis (76-79). Due to the reduction in the incidence of measles disease after the measles-mumps-rubella (MMR) vaccine immunization program in recent years, the measles-induced pancreatitis cases are expected to decline.
Mechanisms involved in viral-induced pancreatitis
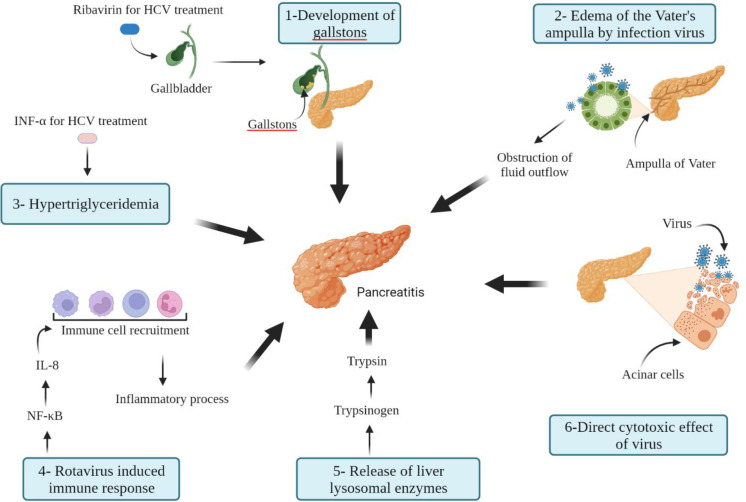
To date, various hypotheses have been proposed to explain viral pancreatitis (Figure 1). A possible mechanism presented for developing HBV-induced acute pancreatitis is the direct cytotoxic effects of the HBV infection on the pancreatic cells. Cavallari et al. demonstrated the presence of HBV-DNA and HBsAg in the cytoplasm of pancreatic acinar cells, indicating the direct destruction of the pancreas by HBV (80). Another possible mechanism may be an HBV-induced immune response which is directed against HBV-infected pancreatic hepatocytes (81). There are various mechanisms by which IFN α may be involved in the development of pancreatitis. Hypertriglyceridemia disorder, a well-known cause of acute pancreatitis, is a result of interferon treatment. From another path, interferon α by stimulating the immune response can lead to a pancreatic-specific autoimmune disease. Also, RBV by causing anemia and chronic hemolysis may lead to gallstones, which is one of the most common causes of acute pancreatitis (19). Development of pancreatitis in patients with HEV and/or acute viral hepatitis (AVH) may result from a multifactorial mechanism. Regarding AVH, one possible explanation is the development of edema of the Vater's ampulla due to viral infection, which leads to obstruction of the outflow of pancreatic fluid. A more probable mechanism is the virus's direct destruction of pancreatic acinar cells. The leakage and circulation of lysosomal enzymes from the damaged liver cells, which change pancreatic trypsinogen to trypsin, may be another possible mechanism of acute pancreatitis.
Figure 1.
An overview of mechanisms involved in the development of acute pancreatitis caused by viral agents
The exact mechanisms by which the Hepatitis A virus causes pancreatitis remain to be elucidated. These mechanisms are the same as those described for the HEV, including direct inflammation and cytotoxic effect of the virus on acinar pancreatic cells and or immune-mediated process against infected pancreatic cells (82). During the VZV infection, the virus may remain latent in the posterior sensory nerve roots, while it contains fibers from both the skin and visceral organs, such as the pancreas. It is assumed that VZV probably damages the membrane of the pancreatic acinar cell and leads to the release of intracellular enzymes. Another possible mechanism is that the patient's immune response causes the cytopathic effect (34). Rotavirus infection has been shown to activate nuclear factor-κB (NF-κB), a critical transcription factor for the up-regulation of interleukin-8 (IL-8) gene expression. IL-8 attracts neutrophils, monocytes, macrophages, and lymphocytes to an inflammatory site, and is involved in the initiation and development of inflammatory responses related to acute pancreatitis (83).
Conclusion
There is extensive information in the literature about the implication of various viral agents in acute pancreatitis and the probable involvement of viruses in the development of acute pancreatitis. However, due to the need for extensive laboratory evidence to determine them as the causative agents of acute pancreatitis, currently, many of these cases are thought to coexist with the occurrence of acute pancreatitis. Nonetheless, if proven that such an association is really true with sufficient evidence, then different aspects related to the diagnosis and treatment of virus-induced acute pancreatitis in patients should be considered. To date, most published studies in this regard have been limited to retrospective and case report studies. Since case reports cannot prove association causality, further studies are required. Also more accurate and advanced diagnostic tests such as PCR, in situ hybridization and biopsy can help us better understand the real role of viruses in causing acute pancreatitis. Future research should establish the most appropriate diagnostic and therapeutic strategies for improving virus-induced pancreatitis outcomes. Last but not least, it is definite that more extensive studies with proper design and precise diagnostic methods are necessary to elucidate the link between viral infections and pancreatic complications.
Conflict of interests
The authors declare that they have no conflicts of interest.
References
- 1.Xiao AY, Tan ML, Wu LM, Asrani VM, Windsor JA, Yadav D, et al. Global incidence and mortality of pancreatic diseases: a systematic review, meta-analysis, and meta-regression of population-based cohort studies. Lancet Gastroenterol Hepatol. 2016;1:45–55. doi: 10.1016/S2468-1253(16)30004-8. [DOI] [PubMed] [Google Scholar]
- 2.Leppäniemi A, Tolonen M, Tarasconi A, Segovia-Lohse H, Gamberini E, Kirkpatrick AW, et al. 2019 WSES guidelines for the management of severe acute pancreatitis. World J Emerg Surg. 2019;14:27. doi: 10.1186/s13017-019-0247-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waller A, Long B, Koyfman A, Gottlieb M. Acute pancreatitis: updates for emergency clinicians. J Emerg Med. 2018;55:769–79. doi: 10.1016/j.jemermed.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 4.Whitcomb DC. Acute pancreatitis. N Engl J Med. 2006;354:2142–2150. doi: 10.1056/NEJMcp054958. [DOI] [PubMed] [Google Scholar]
- 5.Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144:1252–1261. doi: 10.1053/j.gastro.2013.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walling A, Freelove R. Pancreatitis and pancreatic cancer. Prim Care - Clin Off Pract. 2017;44:609–620. doi: 10.1016/j.pop.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Rawla P, Bandaru SS, Vellipuram AR. Review of infectious etiology of acute pancreatitis. Gastroenterol Res. 2017;10:153. doi: 10.14740/gr858w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kundumadam S, Fogel EL, Gromski MA. Gallstone pancreatitis: general clinical approach and the role of endoscopic retrograde cholangiopancreatography. Korean J Intern Med. 2021;36:25. doi: 10.3904/kjim.2020.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klochkov A, Kudaravalli P, Lim Y, Sun Y. Alcoholic pancreatitis. StatPearls [Internet]: StatPearls Publishing; 2021. [PubMed] [Google Scholar]
- 10.De Pretis N, Amodio A, Frulloni L. Hypertriglyceridemic pancreatitis: epidemiology, pathophysiology and clinical management. United Eur Gastroenterol J. 2018;6:649–655. doi: 10.1177/2050640618755002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Müller JA, Groß R, Conzelmann C, Krüger J, Merle U, Steinhart J, et al. SARS-CoV-2 infects and replicates in cells of the human endocrine and exocrine pancreas. Nat Metab. 2021;3:149–165. doi: 10.1038/s42255-021-00347-1. [DOI] [PubMed] [Google Scholar]
- 12.Huo C, Xiao K, Zhang S, Tang Y, Wang M, Qi P, et al. H5N1 influenza a virus replicates productively in pancreatic cells and induces apoptosis and pro-inflammatory cytokine response. Front Cell Infect Microbiol. 2018;8:386. doi: 10.3389/fcimb.2018.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nekoua MP, Bertin A, Sane F, Gimeno J-P, Fournier I, Salzet M, et al. Persistence of coxsackievirus B4 in pancreatic β cells disturbs insulin maturation, pattern of cellular proteins, and DNA methylation. Microorganisms. 2021;9:1125. doi: 10.3390/microorganisms9061125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jain P, Nijhawan S, Rai RR, Nepalia S, Mathur A. Acute pancreatitis in acute viral hepatitis. World J Gastroenterol. 2007;13:5741. doi: 10.3748/wjg.v13.i43.5741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohshiro Y, Tawata M, Takasu N. Acute pancreatitis and exacerbation of hepatitis B following reduced dose of prednisolone. Mon J Assoc Physicians. 2003;96:868–869. doi: 10.1093/qjmed/hcg139. [DOI] [PubMed] [Google Scholar]
- 16.Yuen MF, Chan TM, Hui CK, Chan AOO, Ng IOL, Lai CL. Acute pancreatitis complicating acute exacerbation of chronic hepatitis B infection carries a poor prognosis. J Viral Hepat. 2001;8:459–464. doi: 10.1046/j.1365-2893.2001.00315.x. [DOI] [PubMed] [Google Scholar]
- 17.Alvares-Da-Silva M, Francisconi C, Waechter F. Acute hepatitis C complicated by pancreatitis: another extrahepatic manifestation of hepatitis C virus? J Viral Hepat. 2000;7:84–86. doi: 10.1046/j.1365-2893.2000.00176.x. [DOI] [PubMed] [Google Scholar]
- 18.Tahan V, Tahan G, Dane F, Uraz S, Yardim M. Acute pancreatitis attributed to the use of pegylated interferon in a patient with chronic hepatitis C. J Gastrointestin Liver Dis. 2007;16:224–5. [PubMed] [Google Scholar]
- 19.Chaudhari S, Park J, Anand BS, Pimstone NR, Dieterich DT, Batash S, et al. Acute pancreatitis associated with interferon and ribavirin therapy in patients with chronic hepatitis C. Dig Dis Sci. 2004;49:1000–1006. doi: 10.1023/b:ddas.0000034562.17003.50. [DOI] [PubMed] [Google Scholar]
- 20.Mishra A, Saigal S, Gupta R, Sarin S. Acute pancreatitis associated with viral hepatitis: a report of six cases with review of literature. Am J Gastroenterol. 1999;94:2292–2295. doi: 10.1111/j.1572-0241.1999.01318.x. [DOI] [PubMed] [Google Scholar]
- 21.Raj M, Kumar K, Ghoshal UC, Saraswat VA, Aggarwal R, Mohindra S. Acute hepatitis e–associated acute pancreatitis: a single center experience and literature review. Pancreas. 2015;44:1320–1322. doi: 10.1097/MPA.0000000000000402. [DOI] [PubMed] [Google Scholar]
- 22.Bhagat S, Wadhawan M, Sud R, Arora A. Hepatitis viruses causing pancreatitis and hepatitis: a case series and review of literature. Pancreas. 2008;36:424–427. doi: 10.1097/MPA.0b013e31815d9d53. [DOI] [PubMed] [Google Scholar]
- 23.Sudhamsu K, Khadka S, Sharma D, Chataut S. Acute pancreatitis in acute viral hepatitis. J Nepal Med Assoc. 2011:51. [PubMed] [Google Scholar]
- 24.El-Sayed R, El-Karaksy H. Acute pancreatitis complicating acute hepatitis A virus infection. Arab J Gastroenterol. 2012;13:184–185. doi: 10.1016/j.ajg.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Moleta DB, Kakitani FT, Lima ASd, França JCB, Raboni SM. Acute pancreatitis associated with acute viral hepatitis: case report and review of literature. Rev Inst Med Trop Sao Paulo. 2009;51:349–351. doi: 10.1590/s0036-46652009000600008. [DOI] [PubMed] [Google Scholar]
- 26.Rana SK, Singh R, Aggarwal B, Kumar S. Acute pancreatitis in hepatitis A infection in a 10-year-old boy. Pediatr Infect Dis J. 2013;5:172–174. [Google Scholar]
- 27.Tian Y, Rong L, Nian W, He Y. gastrointestinal features in COVID‐19 and the possibility of faecal transmission. Aliment Pharmacol Ther. 2020;51:843–851. doi: 10.1111/apt.15731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brikman S, Denysova V, Menzal H, Dori G. Acute pancreatitis in a 61-year-old man with COVID-19. Can Med Assoc J. 2020;192:858–859. doi: 10.1503/cmaj.201029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Madhurantakam SR, Arayamparambil PV, Chandan GS, Sarada PP. Acute pancreatitis in COVID-19 patients: two case reports and review of literature. EAS J Anesthesiol Crit Care. 2020;2:165–8. [Google Scholar]
- 30.Samanta J, Gupta R, Singh MP, Patnaik I, Kumar A, Kochhar R. Coronavirus disease 2019 and the pancreas. Pancreatology. 2020;8:1567–1575. doi: 10.1016/j.pan.2020.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inamdar S, Benias PC, Liu Y, Sejpal DV, Satapathy SK, Trindade AJ, et al. Prevalence, Risk factors, and outcomes of hospitalized patients with coronavirus disease 2019 presenting as acute pancreatitis. Gastroenterology. 2020;159:2226–2228. doi: 10.1053/j.gastro.2020.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kulasegaran S, Wilson EJ, Vasquez L, Hulme-Moir M. Varicella zoster virus: a rare cause of acute pancreatitis in an immunocompetent child. Case Rep. 2016;2016:2015213581. doi: 10.1136/bcr-2015-213581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Picod A, Corre E, Maury E, Duriez P, Hoyeau N, Coppo P. Acute pancreatitis in immunocompromised patients: beware of varicella zoster virus primo‐infection. Clin Med Case. 2017;5:1261. doi: 10.1002/ccr3.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roy S, Bose S, Pandey RK, Naramala S, Hossain MR. Acute pancreatitis due to disseminated varicella zoster infection in an individual with newly diagnosed human immunodeficiency virus. Cureus. 2020:12. doi: 10.7759/cureus.7027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Torre JAC, Martín JJD, García CB, Polo ER. Varicella infection as a cause of acute pancreatitis in an immunocompetent child. Pediatr Infect Dis J. 2000;19:1218–1219. doi: 10.1097/00006454-200012000-00025. [DOI] [PubMed] [Google Scholar]
- 36.Franco J, Fernandes R, Oliveira M, Alves AD, Braga M, Soares I, et al. Acute pancreatitis associated with varicella infection in an immunocompetent child. J Paediatr Child Health. 2009;45:547–548. doi: 10.1111/j.1440-1754.2009.01557.x. [DOI] [PubMed] [Google Scholar]
- 37.Kumar S, Jain A, Pandit A. Acute pancreatitis: Rare complication of chicken pox in an immunocompetent host. Saudi J Gastroenterol. 2007;13:138. doi: 10.4103/1319-3767.33467. [DOI] [PubMed] [Google Scholar]
- 38.Maillot C, Riachi G, Francois A, Ducrotte P, Lerebours E, Hemet J, et al. Digestive manifestations in an immunocompetent adult with varicella. Am J Gastroenterol. 1997:92. [PubMed] [Google Scholar]
- 39.Wang Z, Ye J, Han Y-H. Acute pancreatitis associated with herpes zoster: Case report and literature review. World J Gastroenterol. 2014;20:18053. doi: 10.3748/wjg.v20.i47.18053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chan A, Bazerbachi F, Hanson B, Alraies MC, Duran-Nelson A. Cytomegalovirus hepatitis and pancreatitis in the immunocompetent. Ochsner J. 2014;14:295–299. [PMC free article] [PubMed] [Google Scholar]
- 41.Oku T, Maeda M, Waga E, Wada Y, Nagamachi Y, Fujita M, et al. Cytomegalovirus cholangitis and pancreatitis in an immunocompetent patient. J Gastroenterol. 2005;40:987–992. doi: 10.1007/s00535-005-1683-z. [DOI] [PubMed] [Google Scholar]
- 42.Saeed MI, Stephens R, Nwogbo O, Gani IY, Kapoor R, Doroodchi A. Cytomegalovirus pancreatitis in an immunocompetent patient. IDCases. 2020;22:00932. doi: 10.1016/j.idcr.2020.e00932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salazar-Huayna L, Vélez-Segovia E, Ruelas-Figueroa J, Mendo-Urbina F, Montiel-Gonzales M. Cytomegalovirus pancreatitis in immunocompromised patients: a case report. Rev Colomb Gastroenterol. 2014;29:69–73. [Google Scholar]
- 44.Cella P, Gupta S. Diagnosis of cytomegalovirus pancreatitis in AIDS by endoscopic retrograde cholangiopancreatography. N Engl J Med. 1992:326. doi: 10.1056/NEJM199201163260316. [DOI] [PubMed] [Google Scholar]
- 45.Joe L, Ansher A, Gordin F. Severe pancreatitis in an AIDS patient in association with cytomegalovirus infection. South Med J. 1989;82:1444. doi: 10.1097/00007611-198911000-00029. [DOI] [PubMed] [Google Scholar]
- 46.Wislocki LC. Acute pancreatitis in infectious mononucleosis. N Engl J Med. 1966;275:322–323. doi: 10.1056/NEJM196608112750609. [DOI] [PubMed] [Google Scholar]
- 47.Kang S-J, Yoon K-H, Hwang J-B. Epstein-Barr virus infection with acute pancreatitis associated with cholestatic hepatitis. J Pediatr Gastroenterol Nutr. 2013;16:61–64. doi: 10.5223/pghn.2013.16.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kottanattu L, Lava SA, Helbling R, Simonetti GD, Bianchetti MG, Milani GP. Pancreatitis and cholecystitis in primary acute symptomatic Epstein-Barr virus infection–systematic review of the literature. J Clin Virol. 2016;82:51–5. doi: 10.1016/j.jcv.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 49.Shintaku M, Umehara Y, Iwaisako K, Tahara M, Adachi Y. Herpes simplex pancreatitis. Arch Pathol Lab Med. 2003;127:231–234. doi: 10.5858/2003-127-231-HS. [DOI] [PubMed] [Google Scholar]
- 50.Rand K. Isolation of herpes simplex virus type 1 from gastric contents of a patient with acute pancreatitis. South Med J. 1981;74:489–491. doi: 10.1097/00007611-198104000-00030. [DOI] [PubMed] [Google Scholar]
- 51.Olivieri C, Nanni L, Taddei A, Manzoni C, Pintus C. Acute pancreatitis associated with herpes simplex virus infection in a child. Pancreas. 2012;41:330–331. doi: 10.1097/MPA.0b013e3182254a04. [DOI] [PubMed] [Google Scholar]
- 52.Dragovic G. Acute pancreatitis in HIV/AIDS patients: an issue of concern. Asian Pac J Trop Biomed. 2013;3:422–425. doi: 10.1016/S2221-1691(13)60091-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fulco PP, Kirian MA. Effect of tenofovir on didanosine absorption in patients with HIV. Ann Pharmacother. 2003;37:1325–1328. doi: 10.1345/aph.1C412. [DOI] [PubMed] [Google Scholar]
- 54.Martínez E, Milinkovic A, de Lazzari E, Ravasi G, José LB, Larrousse M, et al. Pancreatic toxic effects associated with co-administration of didanosine and tenofovir in HIV-infected adults. Lancet. 2004;364:65–67. doi: 10.1016/S0140-6736(04)16591-4. [DOI] [PubMed] [Google Scholar]
- 55.Moore RD, Keruly JC, Chaisson RE. Incidence of pancreatitis in HIV-infected patients receiving nucleoside reverse transcriptase inhibitor drugs. AIDS. 2001;15:617–620. doi: 10.1097/00002030-200103300-00011. [DOI] [PubMed] [Google Scholar]
- 56.Reisler RB, Murphy RL, Redfield RR, Parker RA. Incidence of pancreatitis in HIV-1–infected individuals enrolled in 20 adult AIDS clinical trials group studies: lessons learned. Acquir Immune Defic Syndr . 2005;39:159. [PMC free article] [PubMed] [Google Scholar]
- 57.Riedel DJ, Gebo KA, Moore RD, Lucas GM. A ten-year analysis of the incidence and risk factors for acute pancreatitis requiring hospitalization in an urban HIV clinical cohort. AIDS Patient Care STDS. 2008;22:113–121. doi: 10.1089/apc.2007.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith CJ, Olsen CH, Mocroft A, Viard JP, Staszewski S, Panos G, et al. The role of antiretroviral therapy in the incidence of pancreatitis in HIV-positive individuals in the EuroSIDA study. AIDS. 2008;22:47–56. doi: 10.1097/QAD.0b013e3282f03094. [DOI] [PubMed] [Google Scholar]
- 59.Nachega JB, Trotta MP, Nelson M, Ammassari A. Impact of metabolic complications on antiretroviral treatment adherence: clinical and public health implications. Curr HIV/AIDS Rep. 2009;6:121. doi: 10.1007/s11904-009-0017-9. [DOI] [PubMed] [Google Scholar]
- 60.Ueda T, Takeyama Y, Yasuda T, Shinzeki M, Sawa H, Nakajima T, et al. Immunosuppression in patients with severe acute pancreatitis. J Gastroenterol. 2006;41:779–784. doi: 10.1007/s00535-006-1852-8. [DOI] [PubMed] [Google Scholar]
- 61.Blum A, Podvitzky O, Shalabi R, Simsolo C. Acute pancreatitis may be caused by H1N1 influenza A virus infection. Isr Med Assoc J. 2010;12:640. [PubMed] [Google Scholar]
- 62.Baran B, Karaca C, Soyer OM, Lacin S, Demir K, Besisik F, et al. Acute pancreatitis associated with H1N1 influenza during 2009 pandemic: a case report. Clin Res Hepatol Gastroenterol. 2012;36:69–70. doi: 10.1016/j.clinre.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 63.Habib A, Jain A, Singh B, Jamshed N. H1N1 influenza presenting as severe acute pancreatitis and multiorgan dysfunction. Am J Emerg Med. 2016;34:1911. doi: 10.1016/j.ajem.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 64.Lampropoulos K, Bazoukis G, Kolyviras A, Tse G, Saplaouras A, Iliopoulos T. Pancreatitis and myocarditis coexistence due to infection by Coxsackie B1 and B4 viruses. Clin Case Rep. 2018;6:23. doi: 10.1002/ccr3.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ozsvár Z, Deák J, Pap A. Possible role of Coxsackie-B virus infection in pancreatitis. Int J Pancreatol. 1992;11:105–108. doi: 10.1007/BF02925981. [DOI] [PubMed] [Google Scholar]
- 66.Tracy S, Höfling K, Pirruccello S, Lane PH, Reyna SM, Gauntt CJ. Group B coxsackievirus myocarditis and pancreatitis: connection between viral virulence phenotypes in mice. J Med Virol. 2000;62:70–81. doi: 10.1002/1096-9071(200009)62:1<70::aid-jmv11>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 67.Giordano S, Serra G, Dones P, Di Gangi M, Failla MC, Iaria C, et al. Acute pancreatitis in children and rotavirus infection. Description of a case and minireview. New Microbiol. 2013;36:97–101. [PubMed] [Google Scholar]
- 68.Parri N, Innocenti L, Collini S, Bechi F, Mannelli F. Acute pancreatitis due to rotavirus gastroenteritis in a child. Pediatr Emerg Care. 2010;26:592–593. doi: 10.1097/PEC.0b013e3181ea72a8. [DOI] [PubMed] [Google Scholar]
- 69.De La Rubia L, Herrera M, Cebrero M, De Jong J. Acute pancreatitis associated with rotavirus infection. Pancreas. 1996;12:98–99. doi: 10.1097/00006676-199601000-00013. [DOI] [PubMed] [Google Scholar]
- 70.Kumagai H, Matsumoto S, Ebashi M, Ohsone T. Acute pancreatitis associated with rotavirus infection. Indian Pediatr. 2009;46:1099–1101. [PubMed] [Google Scholar]
- 71.Nigro G. Pancreatitis with hypoglycemia-associated convulsions following rotavirus gastroenteritis. J Pediatr Gastroenterol Nutr. 1991;12:280–282. doi: 10.1097/00005176-199102000-00024. [DOI] [PubMed] [Google Scholar]
- 72.Hviid A, Rubin S, Mühlemann K. Mumps. Lancet. 2008;371:932–944. doi: 10.1016/S0140-6736(08)60419-5. [DOI] [PubMed] [Google Scholar]
- 73.Tagajdid M, Elkochri S, Elannaz H, Abi R, Amine I. Acute Pancreatitis Caused by Mumps Infection in an Adult. Clin Case Rep Int. 2018;2:1067. [Google Scholar]
- 74.Naficy K, Nategh R, Ghadimi H. Mumps pancreatitis without parotitis. Br Med J. 1973;1:529. doi: 10.1136/bmj.1.5852.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Adler JB, Mazzotta SA, Barkin JS. Pancreatitis caused by measles, mumps, and rubella vaccine. Pancreas. 1991;6:489–90. doi: 10.1097/00006676-199107000-00018. [DOI] [PubMed] [Google Scholar]
- 76.Fusilli G, De Mitri B. Acute pancreatitis associated with the measles virus: case report and review of literature data. Pancreas. 2009;38:478–80. doi: 10.1097/MPA.0b013e31818a3947. [DOI] [PubMed] [Google Scholar]
- 77.Miralbés M, Garreta J, Manonelles A, Gonzalez T, Martinez-Cerezo F. Acute pancreatitis induced by the measles virus: a case report. Pancreas. 1995;11:101–2. doi: 10.1097/00006676-199507000-00011. [DOI] [PubMed] [Google Scholar]
- 78.Morcos N, McHugh H. Pancreatitis associated with measles in a young adult. J Natl Med Assoc. 1997;89:435. [PMC free article] [PubMed] [Google Scholar]
- 79.Rasul KI, Al-Kaabi S. Acute pancreatitis associated with the measles virus. Ann Saudi Med. 2000;20:176–7. doi: 10.5144/0256-4947.2000.176. [DOI] [PubMed] [Google Scholar]
- 80.Cavallari A, Vivarelli M, D'Errico A, Bellusci R, Scarani P, DeRaffele E, et al. Fatal necrotizing pancreatitis caused by hepatitis B virus infection in a liver transplant recipient. J Hepatol. 1995;22:685–90. doi: 10.1016/0168-8278(95)80224-x. [DOI] [PubMed] [Google Scholar]
- 81.Yoo K-S, Lee K-H, Huh KR, Choi WS, Jeon G, Ha J-W, et al. Acute pancreatitis complicating spontaneous acute exacerbation of chronic hepatitis B virus infection: Case report and review of the literature. Gut Liver. 2009;3:64. doi: 10.5009/gnl.2009.3.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thakur A, Basu PP. Acute non-fulminant viral Hepatitis E presenting with acute pancreatitis—an unusual presentation. Malays J Med Sci. 2017;24:102. doi: 10.21315/mjms2017.24.4.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mormile R. Severe gastroenteritis and acute pancreatitis following rotavirus infection in children: The age-related failure of IFN-γ? Immunol Lett. 2016;175:58–9. doi: 10.1016/j.imlet.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 84.D’Incao R, Appel-da-Silva M, Marcon P. Acute hepatitis B leading to acute pancreatitis and acalculous cholecystitis. Gastroenterol Hepatol. 2018;9:34–35. [Google Scholar]
- 85.Cecchi E, Forte P, Cini E, Banchelli G, Ferlito C, Mugelli A. Pancreatitis induced by pegylated interferon alfa‐2b in a patient affected by chronic hepatitis C. Emerg Med J. 2004;16:473–475. doi: 10.1111/j.1742-6723.2004.00653.x. [DOI] [PubMed] [Google Scholar]
- 86.Haffar S, Bazerbachi F, Prokop L, Watt KD, Murad MH, Chari ST. Frequency and prognosis of acute pancreatitis associated with fulminant or non-fulminant acute hepatitis A: a systematic review. Pancreatology. 2017;17:166–175. doi: 10.1016/j.pan.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 87.Haffar S, Bazerbachi F, Garg S, Lake JR, Freeman ML. Frequency and prognosis of acute pancreatitis associated with acute hepatitis E: a systematic review. Pancreatology. 2015;15:321–326. doi: 10.1016/j.pan.2015.05.460. [DOI] [PubMed] [Google Scholar]
- 88.Famularo G, Minisola G, Nicotra GC. Shingles-associated pancreatitis. Pancreas. 2006;33:314–315. doi: 10.1097/01.mpa.0000234076.61254.b2. [DOI] [PubMed] [Google Scholar]
- 89.Kurtovic J, Webster G, Singh‐Grewal I, Bullpitt P, Haindl W, Wakefield D, et al. Acalculous cholecystitis, multifocal gastrointestinal infarction and pancreatitis resulting from Varicella‐zoster virus. Intern Med J. 2005;35:69–70. doi: 10.1111/j.1445-5994.2004.00724.x. [DOI] [PubMed] [Google Scholar]
- 90.Roy P, Maity P, Basu A, Dey S, Das B, Ghosh U. Acute pancreatitis: complication of chicken pox in an immunocompetent host. J Assoc Physicians India. 2012;60:54–5. [PubMed] [Google Scholar]
- 91.Stratman E. Visceral zoster as the presenting feature of disseminated herpes zoster. J Am Acad Dermatol. 2002;46:771–774. doi: 10.1067/mjd.2002.119091. [DOI] [PubMed] [Google Scholar]
- 92.González-Reimers E, Santolaria-Fernádez F, Gómez-Sirvent JL, Méndez-Medina R, Martinez-Riera A. Cytomegalovirus-associated pancreatitis in acquired immunodeficiency syndrome. HPB Surg. 1992;5:181–184. doi: 10.1155/1992/71516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sakakibara Y, Nakazuru S, Kodama Y, Mita E. Acute pancreatitis caused by cytomegalovirus-associated duodenal papillitis. Ann Gastroenterol . 2018:122. doi: 10.20524/aog.2017.0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hammami MB, Aboushaar R, Musmar A, Hammami S. Epstein-Barr virus-associated acute pancreatitis. BMJ Case Rep. 2019;12:231744. doi: 10.1136/bcr-2019-231744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhu Z, Yin S-J, Kong Z-B, Li H, Hu L-P, Zuo S, et al. Pancreatitis combined with Epstein–Barr virus-induced infectious mononucleosis. Chin Med J. 2017;130:2001. doi: 10.4103/0366-6999.211875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zimmerli W, Bianchi L, Oudat F, Spichtin H, Erb P, von Planta M, et al. Disseminated herpes simplex type 2 and systemic Candida infection in a patient with previous asymptomatic human immunodeficiency virus infection. J Infect Dis. 1988;157:597–598. doi: 10.1093/infdis/157.3.597. [DOI] [PubMed] [Google Scholar]
- 97.Konstantinou GN, Liatsos CN, Patelaros EG, Karagiannis SS, Karnesis LI, Mavrogiannis CC. Acute pancreatitis associated with herpes simplex virus infection: report of a case and review of the literature. Eur J Gastroenterol Hepatol. 2009;21:114–116. doi: 10.1097/MEG.0b013e3283005890. [DOI] [PubMed] [Google Scholar]
- 98.Arif M, Nair V, Khan Z. Acute haemorrhagic pancreatitis in HIV-positive patients. S Afr Med J. 2008;98:25–26. [PubMed] [Google Scholar]
- 100.Mortier E, Gaba S, Mari I, Vinceneux P, Pouchot J. Acute pancreatitis during primary HIV-1 infection. Am J Gastroenterol. 2002;97:504. doi: 10.1111/j.1572-0241.2002.05519.x. [DOI] [PubMed] [Google Scholar]
- 101.Rizzardi GP, Tambussi G, Lazzarin A. Acute pancreatitis during primary HIV-1 infection. N Engl J Med. 1997;336:1836–1837. doi: 10.1056/NEJM199706193362516. [DOI] [PubMed] [Google Scholar]
- 102.Sinicco A, Sciandra M, Di Garbo A, Bertone C, Contuzzi E, Raiteri R. Primary HIV infection presenting with acute pancreatitis. Scand J Infect Dis. 1999;31:423–424. doi: 10.1080/00365549950163932. [DOI] [PubMed] [Google Scholar]
- 103.Tyner R, Turett G. Primary human immunodeficiency virus infection presenting as acute pancreatitis. South Med J. 2004;97:393–395. doi: 10.1097/01.SMJ.0000100118.26223.22. [DOI] [PubMed] [Google Scholar]
- 104.Ugwu B, Obekpa O. Acute haemorrhagic pancreatitis in HIV positive patients. West Afr J Med. 2001;20:270–271. [PubMed] [Google Scholar]
- 105.Akuzawa N, Harada N, Hatori T, Imai K, Kitahara Y, Sakurai S, et al. Myocarditis, hepatitis, and pancreatitis in a patient with coxsackievirus A4 infection: a case report. Virol J. 2014;11:1–7. doi: 10.1186/1743-422X-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Coplan NL, Atallah V, Mediratta S, Bruno MS, DePasquale NP. Cardiac, pancreatic, and liver abnormalities in a patient with coxsackie-B infection. Am J Med. 1996;101:325–326. doi: 10.1016/S0002-9343(97)89436-3. [DOI] [PubMed] [Google Scholar]
- 107.Pretagostini R, Quirino L, Pettorini L, Garofalo M, Poli L, Melandro F, et al. Multiple organ failure associated with the coxsackie virus in a kidney transplant patient: case report. Transplant Proc. 2016;48:438–40. doi: 10.1016/j.transproceed.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 108.Taii A, Sakagami J, Mitsufuji S, Kataoka K. Acute Pancreatitis from Mumps Re-infection in Adulthood. JOP. 2008;9:322–326. [PubMed] [Google Scholar]
- 109.Vanlioglu B, Chua TC. Presentation of mumps infection as acute pancreatitis without parotitis. Pancreas. 2011;40:167–168. doi: 10.1097/MPA.0b013e3181eabd3c. [DOI] [PubMed] [Google Scholar]