Figure 1.
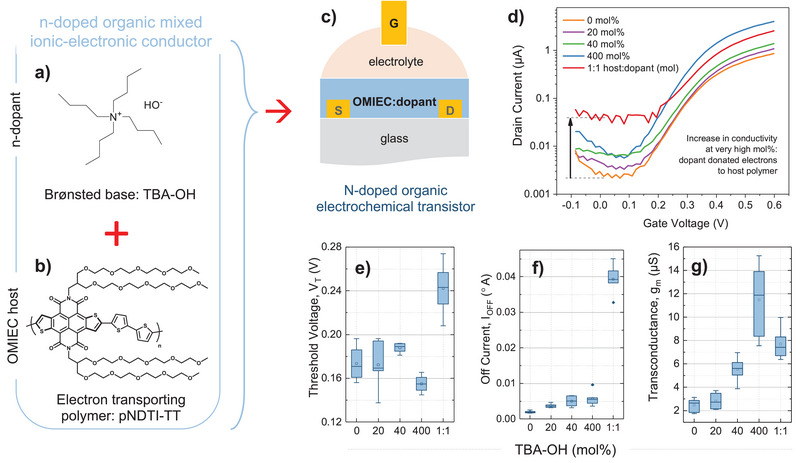
Chemical structure of a) tetrabutylammonium hydroxide (TBA‐OH) and b) naphthodithiophene diimide (pNDTI‐TT), used as the n‐type donor–acceptor copolymer acceptor unit. c) Schematic of a top‐gate bottom‐contact OECT used in this work, indicating that the pNDTI‐TT:TBA‐OH system is used as the organic mixed ionic–electronic conductor active layer. d) Backward sweep transfer characteristics of pNDTI‐TT:TBA‐OH OECTs with 0, 20, 40, 400 mol%, with an additional, highly doped system are included at 1:1. Statistical variation of e) threshold voltage (V T), f) off‐current (I OFF), and g) peak transconductance (g m), with systematically varied quantities of TBA‐OH, taken over six devices each. V T has been extracted from the relationship between √I D and V G. The shift in V T and gradual improvement in g m and I OFF—that worsen again at very high dopant concentrations—indicate that TBA‐OH successfully n‐dopes the pNDTI‐TT. Although the key performance metrics are improved in the best‐performing 400 mol% system, (e)–(g) show a large deviation in the 400 mol% data set. The latter highlights an important point about device uniformity challenges with disordered, solution‐processed polymers, and more so for OECT active layers transporting ions and swelling when exposed to electrolyte. Figure S3 in the Supporting Information confirms the impact of the dopant at 400 mol%: we fabricated an additional six OECTs, on different days and with pNDTI‐TT synthesized in a different batch, to compare the pristine pNDTI‐TT with the best‐performing pNDTI‐TT:TBA‐OH (400 mol%).
