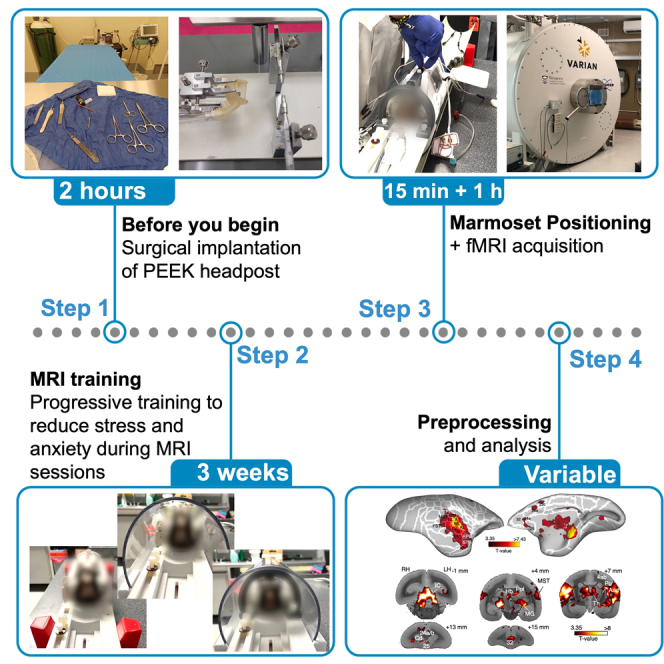
Summary
The common marmoset (Callithrix jacchus) is gaining attention in the field of cognitive neuroscience. The development of an effective protocol for fMRI data acquisition in awake marmosets is a key factor in developing reliable comparative studies. Here, we describe a protocol to obtain fMRI data in awake marmosets using auditory and visual stimulation. We describe steps for surgical and anesthesia procedures, MRI training, and positioning the marmosets within an MRI-compatible body restraint. We then detail fMRI scanning and preprocessing of functional images.
For complete details on the use and execution of this protocol, please refer to Jafari et al. (2023).1
Subject areas: Neuroscience, Cognitive Neuroscience, Behavior
Graphical abstract
Highlights
-
•
Description of an effective protocol for ultra-high-field fMRI in awake marmosets
-
•
Surgical procedure for the implementation of an MRI-compatible PEEK head post
-
•
Marmoset positioning training in MRI-compatible chair for MRI acquisition
-
•
Pipeline for preprocessing and analysis of fMRI images
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
The common marmoset (Callithrix jacchus) is gaining attention in the field of cognitive neuroscience. The development of an effective protocol for fMRI data acquisition in awake marmosets is a key factor in developing reliable comparative studies. Here, we describe a protocol to obtain fMRI data in awake marmosets using auditory and visual stimulation. We describe steps for surgical and anesthesia procedures, MRI training, and positioning the marmosets within an MRI-compatible body restraint. We then detail fMRI scanning and preprocessing of functional images.
Before you begin
The protocol described in this manuscript was used to investigate the brain network involved in the processing of vocalizations of conspecifics in awake common marmosets.1 However, the same protocol has been adapted and used by our research group for multiple experiments, using both acoustic and visual stimuli.2,3,4
Institutional permissions
All experimental procedures described were performed under our Animal Use Protocol approved by Western University’s Animal Care Committee and were conducted in accordance with policies and guidelines put forth by the Canadian Council on Animal Care (CCAC); the Ontario Ministry of Agriculture, Food and Rural Affairs (OMAFRA); and the Animal Research Reporting In Vivo Experiments (ARRIVE). Before you begin, acquire permissions from the relevant institutions.
Head post surgery
Timing: 2 h for surgery + 2–3 weeks for recovery
Before carrying out the steps described in the following protocol, it is necessary to surgically implant a head post onto animals that will be involved in the study. This surgical procedure is similar to that described in detail in previous articles by our research group,5,6 with respect to the implantation of a MRI-compatible head chamber.
-
1.
Administer maropitant citrate (Cerenia, 1 mg/kg) subcutaneously to the marmoset 20 min prior to sedation, to reduce nauseousness, salivation, and bronchial secretions.
-
2.
Induce anesthesia with intramuscular injection of ketamine (15–20 mg/kg) plus medetomidine (0.025 mg/kg) mixed in the same syringe.
-
3.
Place a catheter (gauge 24) in one of the lateral tail veins.
-
4.
Via a syringe pump, perform a continuous rate infusion of propofol (0.3–0.5 mg/kg) mixed 50–50 in 0.9% saline solution.
-
5.
To maintain general anesthesia during the surgical procedure, deliver gaseous isoflurane (0.5%–3.0%) in oxygen via a custom-designed 3D printed mask (see key resources table for CAD file), attached to the stereotactic frame.
-
6.
To combat inflammation, give a loading dose of the NSAID meloxicam (0.2 mg/kg) subcutaneously.
-
7.
Deliver intravenously an antibiotic Cefazolin (22 mg/kg) diluted by 10 in 0.9% saline solution at the beginning of the surgical procedure and every 90 min.
-
8.
Place a small amount of sugar paste under the animal’s tongue to prevent hypoglycemia during the surgery.
-
9.
Maintain body temperature using a circulating water blanket, a forced-air warming blanket, and fleece blankets placed under the sterile surgical drape.
-
10.
Monitor the temperature, heart rate, respiration rate, blood pressure, and SpO2 throughout surgical procedure.
-
11.
Place the animal in the stereotactic frame (Narishige, Model SR-6C-HT).
-
12.
Perform a midline incision along the cranium and retract the temporalis muscle to gain access to the underlying periosteum.
-
13.
Carefully remove the periosteum and any other residual tissue from the skull. To accomplish this, clean the skull surface using hydrogen peroxide (2%) and cotton swabs.
Note: Since the skull of the common marmoset is small and extremely fragile, the use of fixation screws is not recommended. Furthermore, the small size of these animals is well suited to the use of fixation methods based only on adhesive strength. For this reason, we have employed a surgical procedure which involves the use of cement typically used in dental restoration. However, to ensure the effectiveness of this type of implant, it is critical to carefully prepare the surface of the skull to achieve optimal bonding of the dental cement.
-
14.
Stop any residual bleeding around the skull using 15.5% ferric sulfate hemostatic gel (or equivalent).
-
15.
Using a small metal brush, gently abrade the skull surface.
-
16.
Rinse the skull surface thoroughly with sterile saline, then dry thoroughly all fluids and remove all bone residue using absorbent cotton swabs.
-
17.
Using a microbrush (Bisco disposable regular applicator, item number: X-80250P; Bisco Schaumburg, Illinois, USA), apply two layers of adhesive resin (All-bond Universal, Bisco, Schaumburg, Illinois, USA) on the skull.
-
18.
Air dry the resin (you can use a sterile empty 30 cc syringe).
-
19.
Cure the surface with an ultraviolet dental curing light (Dental Power).
-
20.
Place the head post on the stereotactic manipulator, ensuring its correct orientation and position on the marmoset’s head.
Note: The head post is designed based on a computerized tomography (CT) scan of a marmoset head. To determine the correct position of the head post, locate the vertex of the marmoset's skull, which is situated halfway between the nasion and inion (rostral-caudal direction) and between the right and left pre-auricular points. The center of the head post, where the fastening screw is present, should coincide with this vertex.
-
21.
Apply the resin composite (Core-flo DC Lite, Bisco, Schaumburg, Illinois, USA) on the layer of adhesive resin and on the bottom of the head post.
Note: Our head post is machined from solid polyetheretherketone, or PEEK, plastic. You can find a representation of this head post in Figure 1C and the related CAD file has been made publicly available (see key resources table for source). In the base of our head post we designed several small holes, to allow the cement to fill in during the surgery and ensure better adhesion to the skull. When the marmosets are not imaged or in training, a PEEK screw is inserted in the head post, to keep debris from entering the screw threads.
-
22.
Lower the head post onto the skull using the stereotactic manipulator.
-
23.
Cure the surface using the ultraviolet dental curing light (Dental Power).
-
24.
Using a fine tip applicator, fill the holes in the base of the head post to ensure a better adhesion to the skull.
-
25.
Smoothen the adhesive cement around the external edges of the head post, as sharp edges may irritate or lacerate the surrounding tissues.
-
26.
Cure the adhesive with ultraviolet dental curing light (Dental Power).
-
27.
To ensure a tight contact between the cement surface and the edges of the wound, gather the caudal and rostral ends of the incision with wound clips (Fine Science Tools).
-
28.
Post-operatively, administer buprenorphine (0.005–0.01 mg/kg) intramuscularly once the animal starts to wake up, then repeat after 6–8 h post-surgery.
-
29.
Administer Meloxicam (0.1 mg/kg) orally once a day for 5 days for pain management plus infants Acetaminophen (10–20 mg/kg) orally twice daily for 5 days.
Pause point: To ensure good adherence of the head post and to allow the animal to recover following the surgical operation, it is advisable to wait at least 2–3 weeks before starting the MRI training inside the body restraint. During this period, check the animal health and in particular the skin edges approximating the dental cement must be reviewed on a daily basis at minimum.
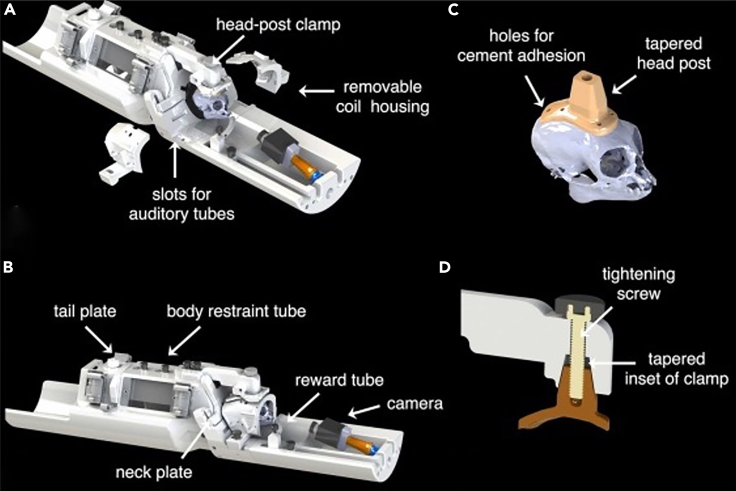
Figure 1.
Detailed representation of the body restraint system and the PEEK headpost
(A) CAD drawing of the body restraint used in this paper and fully described in Gilbert et al., 2023.7 Initially secure the marmoset in the body restraint using the neck plate. Subsequently, depending on the size of the animal, place within the body restraint a back plate of variable size and close the tail plate. Finally, screw the clamp first to the head-post and secondly to the body restraint. Once the monkey is fixed, earphones (if necessary) or silicone earplugs to reduce the gradient noise during the fMRI sessions may be positioned.
(B) After the steps described in A, screw the two parts of the coil onto the dedicated slots in the frontal part of the body restraint.
(C and D) (C) Representation of the position and size of the head post applied on the marmosets’ skull. To fixate the animal’s head to the body restraint, position the clamp onto the head post, and then secure it with a PEEK screw, as shown in D. This procedure will draw the head post into the clamp, which can finally be secured to the body restraint. From ref.7 with permission.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Deposited data | ||
| Analyzed data (individual and group levels), auditory stimuli | Jafari et al.1 | https://zenodo.org/record/7786440#.ZCb1my8r1z8 |
| Computer-aided design (CAD) files of the restraint system, RF coil, back plate, and PEEK head post | Gilbert et al.7 | http://doi.org/10.17605/OSF.IO/VTQEP |
| CAD file of the 3D printed anesthesia mask | This study | https://doi.org/10.5281/zenodo.8045220 |
| Experimental models: Organisms/strains | ||
| Common marmoset (Callithrix jacchus), adult (>24 months old) | University of Western Ontario, 1151 Richmond Street, London, ON, Canada, N6A 5B7 | N/A |
| Software and algorithms | ||
| Matlab_R2022a | MathWorks | https://www.mathworks.com/products.html?s_tid=gn_ps |
| Audio scrambling | Ellis et al.8 | https://www.mathworks.com/matlabcentral/fileexchange/29396-time-domain-scrambling-of-audio-signals?s_tid=srchtitle |
| AFNI: Analysis of Functional NeuroImages (v21.3.05) | Cox et al.9 | https://afni.nimh.nih.gov/pub/dist/doc/htmldoc/background_install/main_toc.html |
| FSLeyes | Smith et al.10 | https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSLeyes |
| Connectome Workbench (v1.5.0) | Marcus et al.11 | https://www.humanconnectome.org/software/get-connectome-workbench#download |
| Advanced Normalization Tools (ANTs) | Avants et al.12 | http://stnava.github.io/ANTs/ |
| Python v3.11.0 | Python Software Foundation | https://www.python.org |
| LabChart | ADInstruments | https://www.adinstruments.com/products/labchart |
| Code described in this paper | This study | https://zenodo.org/record/7789270 |
| Other | ||
| MRI scanner – horizontal 31 cm bore, 9.4 T | Agilent | 9.4 T/310/ASZ |
| Gradient coil, 15 cm diameter, 400 mT/m max gradient strength | Handler et al.13 | N/A |
| Eight-channel receive coil | Gilbert et al.7 | N/A |
| MRI-compatible earphones | Sensimetrics, Gloucester, MA | https://www.sens.com/products/model-s14/ |
| Sound-attenuating silicone earplugs | Amazon | https://www.amazon.ca/s?k=silicone+ear+plugs+pillow&crid=7B5T499BF24P&sprefix=silicone+ear+plugs+pillow%2Caps%2C86&ref=nb_sb_noss_2 |
| MRI-compatible camera, 60 Hz | MRC Systems GmbH, Germany | Model 12M-i |
| LCSD projector | Sony | Model VLP-FE40 |
| Raspberry Pi | Raspberry Pi Foundation | Model 3B+ |
| Respiratory pillow | SA Instruments, Inc | https://i4sa.com/product/model-1025t-monitoring-gating-system/ |
| Stereotactic frame | Narishige | Model SR-6C-HT |
| Disposable microbrush | Bisco Schaumburg, Illinois, USA | Item number: X-80250P |
| Ultraviolet dental curing light | Dental Power | Dental Wireless Cordless LED curing light, reference: lux60 |
| Resin composite | Bisco, Schaumburg, Illinois, USA | Core-flo DC Lite, item number: A-17801P |
| Syringe pump | SAI Infusion Technologies | Baxter AS50 Pump – refurbished |
| Pump for water blanket | Palm Industrial, Canada | Gaymar T Pump 07999-000 |
| Pump for forced-air blanket | Southwest Medical, Oklahoma, USA | 3M Bair Hugger Model 505 Patient Warming System |
| Metal brush | Gesswein Canada | Supra ME wire end brushes, straight wire, item number 110-8791 |
| Wound clips (9 mm) | Fine Science Tools | Item number 12022-09 |
Materials and equipment
fMRI setup
We collected all the data here described using a 9.4 T 31-cm horizontal bore magnet and a Bruker BioSpec Avance III HD console running the Paravision 7 software package. We used a custom-built gradient coil with a 15-cm inner diameter and a maximum gradient strength of 430 mT/m13 coupled with eight separate receive channels.7 These channels were connected to preamplifiers (Siemens Healthcare), located behind the animal, and to the receive coil, placed inside an in-house built quadrature birdcage coil (12-cm inner diameter) used for transmission.
In experiments using auditory stimuli (as in ref.1), we suggest using a continuous acquisition paradigm including silent periods (“sparse” paradigm, already described in the literature14) in order to limit the potential masking of the experimental stimuli by the scanner noise. In this type of paradigm, the slices are acquired in less time than the repetition time (TR), thus leaving a silent interval during which the noise of the scanner is strongly reduced, and it is possible to perceive the auditory stimuli without interference. In our auditory experiments, we acquired the functional images using a gradient-echo-based, single-shot echo-planar imaging (EPI) sequence with the following parameters: TR = 3 s, acquisition time TA = 1.5 s, TE = 15 ms, flip angle = 40°, field of view = 64 × 48 mm2, matrix size = 96 × 128, voxel size = 0.5 mm isotropic, number of slices = 42 [axial], bandwidth = 400 kHz, GRAPPA acceleration factor = 2 (left-right). In experiments using only visual stimuli the noise of the scanner is not a concern (given that all animals have silicone earplugs to reduce this noise). Thus, our usual EPI sequence in these cases is identical to the previous one, but reducing the TR to 1.5 s, making the repetition time and the acquisition time coincide.2,3
In both cases, in each fMRI session we collected a second set of EPIs using the opposite phase-encoding direction (right-left) to correct the functional images for the spatial distortion caused by non-uniformities in the main magnetic field.
Finally, it is necessary to acquire an anatomical image per animal, in order to perform the anatomical registration of the functional data. In a separate scan, we thus acquired a T2-weighted structural image with the following parameters: TR = 7 s, TE = 52 ms, field of view = 51.2 × 51.2 mm2, voxel size = 0.133 × 0.133 × 0.5 mm3, number of slices = 45 [axial], bandwidth = 50 kHz, GRAPPA acceleration factor: 2.
Physiological monitoring setup
The small diameter of preclinical 9.4 T MR scanners makes it difficult to monitor the condition of the animal once the body restraint is inserted into the bore. Our protocol does not include anesthesia of the marmosets, reducing the risk of a too deep or too light sedation. However, strong anxiety, manifestations of distress or an erroneous position of the animal within the body restraint can represent a danger to the monkey health and/or a confounding factor at the time of data analysis. It is therefore advisable to monitor the physiological state of the animal during the experimental sessions. In our experimental setup we positioned an MRI-compatible camera (60 Hz, MRC Systems, model 12M-i) in front of the marmoset (see Figures 1A and 1B). By connecting this video camera to a screen external to the magnet room, it is possible to constantly monitor the animal’s state of wakefulness and any motor agitation. Furthermore, through the use of a respiratory pillow, we can check the animal’s respiratory rate, important indicator of a potential state of anxiety or stress. By connecting this sensor to a computer equipped with LabChart (SA Instruments), a veterinary technician can check the regularity of these signals during the entire experimental session.
Note: Similar to humans, marmosets can exhibit differences in their sensitivity to magnetic fields, being more or less susceptible to symptoms such as nausea or nystagmus. These symptoms tend to reduce until they disappear within a few minutes, and it is therefore advisable to wait for the animal's state to stabilize before starting the acquisition of functional images. To minimize these symptoms, insert the body restraint very slowly inside the magnet, allowing for progressive adaptation to the magnetic field.
CRITICAL: In the case of apparent nausea (as indicated by swallowing or licking behaviors) followed by vomiting, constantly and scrupulously check the video provided by the MRI-compatible camera and the respiratory rhythm. Given the prone position and the fixation of the head at an angle, excessive and repeated vomiting could prevent the animal from breathing properly, resulting in aspiration, and putting its health at risk. In this case of repeated regurgitation, increasing respiratory rate and/or clear motor agitation of the animal, remove the animal from the scanner using the slowest possible movement consistently with the risk the situation represents for the monkey: it is preferable to increase the speed of this procedure if the state of the animal deteriorates rapidly.
Step-by-step method details
MRI training
Timing: 3 weeks
To reduce the animal’s stress and anxiety during the experimental sessions, carry out a three-week acclimatization procedure.
-
1.During the first week, restrain the marmoset within the body restraint, without fixating the head, starting at 10 min and increasing the interval up to 30 min.Note: A representation of the body restraint we used is available in Figures 1A and 1B (from ref.7). This was entirely designed and 3D-printed in-house; the CAD files and full details have been made public (see key resources table).
-
a.Let the animal enters the tube from the rear entrance until its head emerges from the anterior hole.
-
b.Secure this position by lowering the neck plate.
-
c.Once the plate is closed, and the animal cannot withdraw into the tube, place a back plate of variable size in the tube, above the animal’s back, to further reduce the animal’s movements by filling the dead space.Note: The size of this plate depends on the size of the monkey. For this first step, the animal remains in this position without any other type of stimulus or constraint (see Figure 2).Note:Methods videos S2 and S4 depicts the steps needed to get the animal into the body restraint, close the neck and tail plates and place the back plate.
-
a.
-
2.During the second week, restrain the animal inside the body restraint and then insert this latter inside a mock-MRI tube (a simple plexiglass tube, with a diameter sufficient to allow the animal holder to enter, can serve this purpose: see Figure 3).
-
a.Without fixating the head, play gradient-coil sounds, increasing their volume up to 80 dB, to simulate the noise that animals will hear during the experimental sessions.Note: This sound level is actually lower than the noise of our ultra-high-field MRI scanner at 9.4 T, but during the experimental sessions this noise will be muffled by silicone earplugs.
-
b.Increase the duration of this training gradually by 10 min, up to 60 min.
-
a.
-
3.
In the last week of training, carry out the same steps described for the first two weeks, but fixing the animal’s head post (represented in Figure 1C) to the appropriate head-post clamp (see Figure 1D). See Figure 4.
CRITICAL: To validate each step of the training and move on to the next, the animal must exhibit relatively calm behavior. It is advisable to reward the animals during training sessions (with small portions of pudding or marshmallow fluff for example) so the animals face forward and reduce their movement. To successfully complete the training, each animal must achieve a score of 1 or 2 on the behavioral assessment scale developed by Silva and co-workers.15
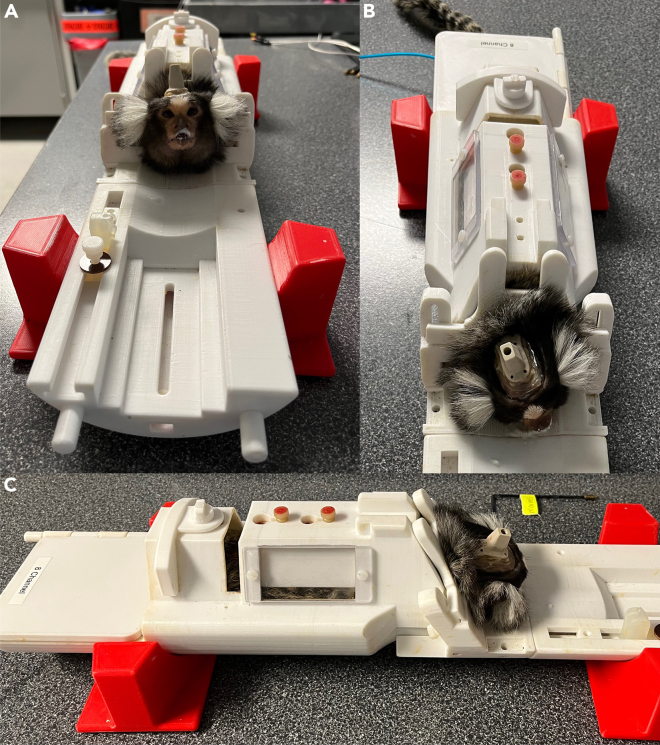
Figure 2.
First week of MRI training: Familiarization with the body restraint
(A–C) Frontal (A), superior (B) and lateral (C) views of the setting used for the first week of MRI training. During this week, the marmoset’s head is not fixed to the body restraint and no gradient-coil sounds are played.
Figure 3.
Second week of MRI training: Gradient-coil sounds in the mock-MRI scanner
(A–C) Frontal (A), superior (B) and lateral (C) views of the setting used for the second week of MRI training. During this week, the marmoset’s head is not fixed, but the body restraint is positioned inside a mock-MRI scanner and gradient-coil sounds are played during the session.
Figure 4.
Third week of MRI training: Head fixation
(A–C) Frontal (A), superior (B) and lateral (C) views of the setting used for the last week of MRI training. During this week, the marmoset’s head is fixed to the body restraint via the head-post clamp. The body restraint is then positioned inside the mock-MRI tube and gradient-coil sounds are played during the session.
Marmoset positioning before fMRI session
Timing: 10–15 min
This section describes how to position the marmoset inside the body restraint before the fMRI session. All these steps are performed outside the MRI scanner room, in the adjacent preparation room. You need to prepare the room with all the necessary materials before the marmoset arrives, to avoid long times of preparation, which would result in higher stress for the animal being in an unusual room.
-
4.Place the respiratory pillow around the marmoset’s chest (see Methods video S1).
-
a.While one experimenter holds the animal below the shoulders and at thigh level, exposing the thorax, the second experimenter wraps the respiratory pillow containing the sensor capable of measuring respiratory rate.
Methods video S1. Procedure to correctly place the respiratory pillow around the marmoset’s chest, related to Step 4Download video file (24.8MB, mp4) -
a.
Note: Secure the pillow with tape to stabilize the sensor, ensuring it remains in place during any subsequent manipulations. However, be careful to not impede the marmoset's breathing.
-
5.Let the marmoset enter the body restraint.
-
a.Holding the animal with both hands, insert the head into the rear opening of the body restraint, paying attention that the head post does not hit or get stuck in the holes dedicated to the tail plate.Note: As it is possible to observe in the Methods videos S2, S3, S4, S5, S6, S7, and S8, our experimental setup uses a cradle to load the animal within the body restraint into the scanner during the MRI sessions. The body restraint is positioned within the crate throughout all the steps described above, to keep it stable during the positioning procedure. However, the edges of the cradle may represent an obstacle during setup. To avoid this, use a support that raises slightly the body restraint from the cradle. This step can facilitate the procedure but does not affect positioning of the animal inside the body restraint. The support we use can be seen in the Methods video S6.Methods video S2. Practical implementation of the steps necessary to correctly position the marmoset within the MRI-compatible body restraint, related to Step 5a-cDownload video file (42.8MB, mp4)Methods video S3. Practical implementation of the steps necessary to correctly fix the marmoset’s head using the four-points fixation system, related to Step 5d-fDownload video file (45.9MB, mp4)Methods video S4. Practical implementation of the steps necessary to place the back plate within the body restraint and subsequently close the tail plate, related to Step 5g-iDownload video file (47MB, mp4)Methods video S5. Practical implementation of the steps necessary to correctly position the earphones encased in the silicone earplugs, related to Step 5j-kDownload video file (44.5MB, mp4)Methods video S6. Practical implementation of the steps necessary to secure the two removable coil housing parts and match the body restraint with the isocenter, related to Step 5l-mDownload video file (42.7MB, mp4)Methods video S7. Practical implementation of the steps necessary to connect the eight channels of the coil to the pre-amplifiers, related to Step 5n-oDownload video file (30.8MB, mp4)Methods video S8. Practical implementation of the steps necessary to secure the two earphones once the head of the marmoset is fixed, related to problem 2Download video file (43.6MB, mp4)
-
b.Gently push the animal into the body restraint with one hand, using the other hand to hold the neck plate open, thus allowing the head to exit. If the marmoset tries to resist this procedure, see troubleshooting 1.
-
c.While still restraining the animal with one hand, close the neck plate and tighten the two side screws, thus preventing the marmoset from backing up in the body restraint (see Methods video S2).Note: Check that the closure of the neck plate is not pinching the skin of the animal's neck or shoulders.
-
d.Remove the protective screw contained within the head post.
-
e.Insert the head post into the appropriate cavity of the head post clamp, then use the PEEK screw to connect the head post to the clamp.
-
f.Screw in the four screws (two front side, two rear) which allow you to finalize the fixation of the marmoset’s head.Note: The head fixation system shown in the video and described here is a modification of what is shown in Figure 1. The previous model, with only two fixation screws, located on the rearmost part of the head post clamp, allowed animals endowed with greater strength to make small vertical movements of the head. In order to reduce this movement, the new version (represented in Methods video S3) includes two additional fixation screws, located in front of the animal's head. This four-points fixation system eliminates any form of head movement, even by the strongest animals. The only difference in this protocol, if using the old version, pertains to this step: instead of using the 4 screws, only the two rearmost screws will be used.
-
g.Select the back plate that best fits the size of the marmoset.
-
h.Secure the back plate with the two appropriate screws.Note: To find out which back plate is the best choice, insert your hand through the back opening, passing over the marmoset's back. Flatten the animal's back and observe the space between it and the upper surface of the body restraint. If in doubt, start with the smallest back plate and try to fit your hand. The ideal back plate prevents the animal from arching its back but at the same time does not apply pressure on the marmoset.
-
i.Close the tail plate.Note: The body restraint has two openings for the tail plate. Depending on the size of the animal, the tail plate can be placed in the most posterior slot, for bigger marmosets, or in the more anterior slot. Check during the procedure which slot best fits the body length of the marmoset inside the body restraint.Optional: In the Methods video S4 it is possible to observe the presence of a folding tail cover, which can be closed after the positioning of the tail plate. This element is not essential for the good fixation of the animal, but it is effective in limiting the movements of the tail. Continuing in the video, it is indeed possible to observe that the cables of the 8 channels of our coil pass a few centimeters above the tail, and the movements of the tail can therefore hit the cables or the connectors. Also, a long tail may become entangled with the cables, causing signal interference. To avoid all this, it is possible to provide a tail cover which can be closed at the end of the procedure, to limit the movements of the tail. In the case of animals particularly inclined to wag their tails, it is advisable to add a piece of adhesive tape to this cover, to prevent accidental reopening.
-
j.Encase the ends of the two insert earphones with silicone earplugs, creating a protective layer that will shield the ear from the scanner’s noise.Note: These earphones are small enough to fit the marmoset’s ears, and they are connected with a transformer and an audio amplifier. The latter receives the auditory signal directly from a computer, though a common jack connector.
-
k.Place the first earphone in one ear, keeping it in place by shaping the silicone inside the ear of the marmoset. Once the first is secured, move on to the second earphone (see Methods video S5).Note: If the experiment does not include auditory stimuli, the earphones are not necessary. Thus, skip step j and position the silicone earplugs in the ears of the marmoset, modelling them to the shape of the auricle.
 CRITICAL: Do not put pressure directly on the earphone to try to hold it in place, but rather press on the surrounding silicone. Excessive pressure on the earphone could push it too far into the animal's ear, damaging the eardrum. If the silicone is not sufficient to hold the earphone in place, proceed to troubleshooting 2.
CRITICAL: Do not put pressure directly on the earphone to try to hold it in place, but rather press on the surrounding silicone. Excessive pressure on the earphone could push it too far into the animal's ear, damaging the eardrum. If the silicone is not sufficient to hold the earphone in place, proceed to troubleshooting 2. -
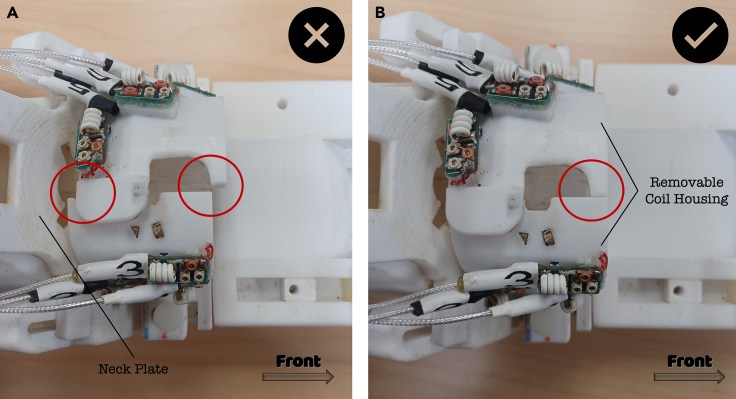
l.Place the two removable coil housing parts, securing them with the two suitable screws. Check that the frontal border of the two parts is at the same level and not shifted posteriorly/anteriorly (see Figure 5), as this shift may influence the quality of the signal.
-
m.Remove the support below the body restraint, then slide the latter until the mark indicated on the coil housing parts matches the isocenter position marked on the cradle (see Methods video S6).Note: This step depends on the experimental setup present in the laboratory. In our case, the cradle is inserted inside the scanner up to a predetermined position, and it is therefore possible to mark the position of the isocenter on the cradle. Matching the body restraint with the isocenter is therefore relatively straightforward. Different experimental setups may include different methodologies, different cradles or even the absence of a cradle (especially in the case of larger diameter scanner bores, as in the case of a 7T or 3T scanner).
-
n.Position the pre-amplifiers behind the body restraint.
-
o.Connect the eight channels of the coil to their respective connectors (see Methods video S7).Note: All the steps described here, except for step 4 (respiratory pillow), can be performed by a single experimenter. We suggest, however, that two people take care of positioning the animal inside the body restraint, to speed up the procedure and reduce the animal's stress period. In the case of two people, we suggest that the division of tasks is done on a spatial basis: one experimenter can take care of the tasks related to the "posterior" part of the body restraint (insert the animal, apply the back plate, close the tail plate, position the pre-amplifiers and connect the cables related to the eight channels). The second experimenter can take care of the "frontal" part of the body restraint (close the neck plate, remove the protective screw from the head post, fixate the marmoset head, position the earphones/silicone earplugs and screw the two parts of the coil into the appropriate slots). However, this distribution of tasks can be modified as needed.
-
p.Push the cradle and the body restraint slowly into the magnet bore until the predetermined position is reached and the marmoset’s head is at the isocenter.
-
q.Connect the cables of the MRI-compatible camera and the respiration pillow to the correspondent devices.
-
r.Monitor the state of the animal, both visually through the images provided by the MRI-compatible camera, and by checking the physiological signal transmitted by the respiratory pillow.
-
a.
Figure 5.
Detailed view of the removable coil housing positioning
(A and B) Representation of an erroneous (A) and a correct (B) position of the two removable coil housing parts. The frontal-posterior shifting of the two parts may negatively affect the quality of the signal during the MR scan.
fMRI acquisition
Timing: 1 h
This section describes the steps to record BOLD signal changes in awake marmosets using a 9.4 T Bruker scanner (see key resources table).
-
6.
Localize the marmoset’s head and brain through a standard localizer, checking they match the position of the isocenter.
-
7.
Calibrate the power of the transmit RF coils.
-
8.
Optimize the homogeneity of the magnetic field using a MAPSHIM shimming protocol.
Note: Positioning the head of the marmosets in the isocenter should improve the efficacy of B0 shimming and reduce inter-animal variations in spatial distortion. However, in some sessions it is possible to observe highly distorted images or low signal after B0 shimming. If this is the case, see Troubleshooting 3.
Expected outcomes
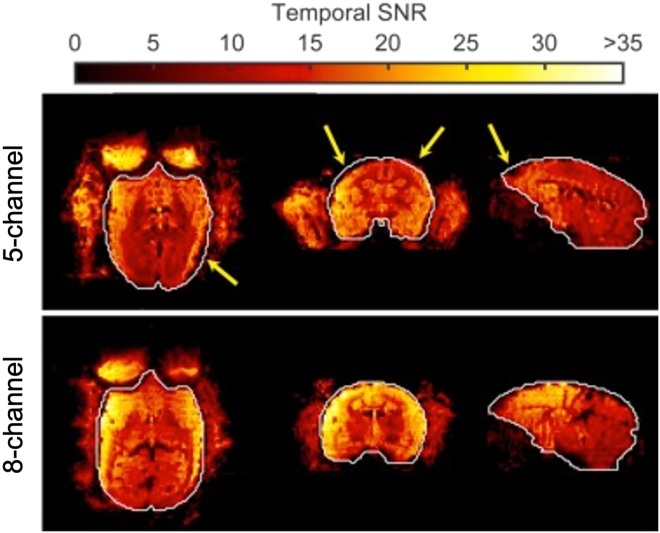
We have described the necessary steps to acquire fMRI data using ultra-high (9.4 T) magnetic field in fully awake common marmosets. Through the surgical implantation of an MRI-compatible head post and the subsequent three-week training, aimed at reducing the animal’s stress during the experimental sessions, our procedure allows us to minimize motion artifacts during the acquisition of functional images. Furthermore, our 8-channel coil produces a higher temporal signal-to-noise ratio (tSNR) than that produced by our previous 5-channel version, especially at the periphery of the brain (see Figure 6 for a detailed comparison between the two different types of coils, see ref;7). The tSNR is a measure that allows for the assessment of SNR during a functional time-course, where physiological fluctuations can represent a significant noise source. A higher SNR therefore corresponds to higher image quality during functional runs.
Figure 6.
Representation of the temporal SNR produced by a 5-channel (top row) and our 8-channel coil (bottom row), obtained during a two-fold accelerated EPI acquisition with 500-μm isotropic resolution
The 8-channel coil produces higher tSNR across the entire brain, with the highest gains in the periphery. White lines delineate the boundaries of an undistorted, co-registered anatomical image. Yellow arrows delineate regions in which the 5-channel coil produced a signal dropout or greater distortion. Adapted with permission from ref.7
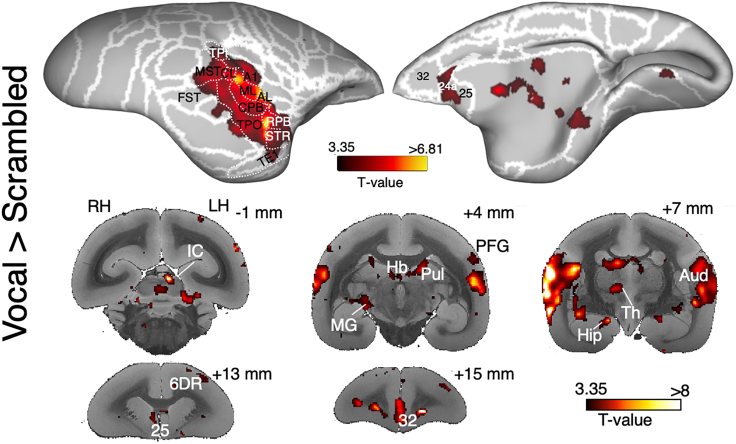
Thanks to this procedure, our research group was able to map multiple functional circuits of the common marmoset, finding confirmations of the results obtained both in the electrophysiological literature on this small New-World primate and in the comparative literature on Old-World macaques and humans. An example of such results can be seen in Figure 7 (adapted from ref.1). In this study, we presented 9 awake marmosets (with 6 to 14 functional runs per animal) with three categories of auditory stimuli: marmosets' vocalization, their phase-scrambled version, and non-vocal sounds. The effectiveness of our experimental setup can be seen in the comparison between these categories: as it is possible to observe in Figure 7, the comparison between marmosets' vocalizations and their scrambled version induced stronger activations at both cortical and subcortical level.
Figure 7.
Functional activations for the comparison between marmosets’ vocalizations and scrambled vocalizations
T-maps are thresholded at T = 3.35 (p < 0.01 uncorrected) and displayed on the right fiducial brain surface (lateral and medial view). White lines delineate the brain parcellation based on the Paxinos marmoset’s atlas.16 Coronal slices of anatomical MR images are used to depict subcortical activations. Adapted from ref.1 with permissions. TPt: temporoparietal transitional area; MST: medial superior temporal area; FST: fundus of superior temporal sulcus; A1: primary auditory cortex; CL: caudolateral auditory cortex; ML: middle lateral auditory cortex; CPB: caudal parabelt auditory area; RPB: rostral parabelt area; AL: anterolateral auditory area; TPO: temporo-parieto-occipital association area; STR: superior temporal rostral area; 6DR: dorsorostral area 6; IC: inferior colliculus; Hb: habenula; Pul: pulvinar; MG: medial geniculate nucleus; Th: thalamus; Hip: hippocampus.
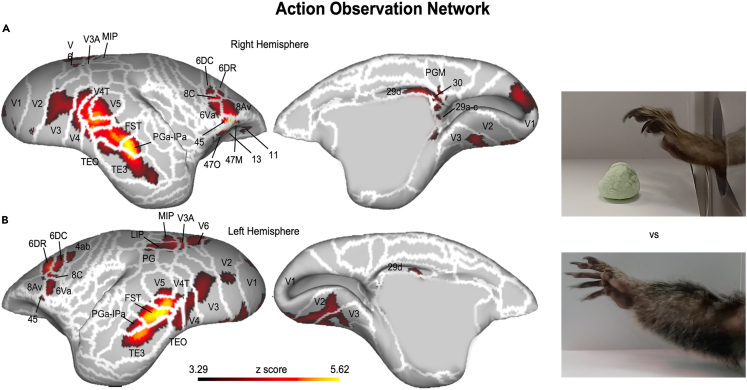
Another possibility offered by our experimental setup concerns the use of visual stimuli. In a recent study, we presented 7 awake common marmosets (10 functional runs per animal) with videos depicting goal-directed (i.e., grasping a marshmallow) or non-goal-directed (i.e., grasping the air) actions.3 The objective of this study was to investigate the action observation network, a large-scale network of brain areas that responds during the observation of goal-directed actions performed by other individuals around us. This network, in macaques and humans, includes areas of the superior temporal sulcus, inferior parietal region, and premotor/prefrontal cortex. Our results (depicted in Figure 8) confirm the existence of such a network also in the common marmoset and further validate our experimental protocol, revealing an action observation network showing large overlap with the humans and macaque network. This network includes occipitotemporal, parietal, and premotor/prefrontal activations, plus subcortical activations in the hippocampal formation and thalamus.
Figure 8.
Functional activations for the comparison between goal-directed and non-goal-directed actions
(A and B) Z-maps are thresholded at z = 3.29 (corresponding to p < 0.001) and survived the cluster-size correction (10,000 Monte-Carlo simulations, α = 0.05). Results are displayed on the left (A) and right (B) fiducial brain surfaces (both on lateral and medial view). White lines delineate the brain parcellation based on the Paxinos marmoset’s atlas.16 From ref.3 with permission. 6Va: ventral part a of area 6; 6DC: dorsocaudal area 6; 6DR: dorsorostral area 6; FST: fundus of the superior temporal sulcus.
Quantification and statistical analysis
For the sake of the present manuscript, we describe here the steps performed in the preprocessing and statistical analyses of the functional images obtained at ultra-high magnetic fields from awake common marmosets using an auditory stimulation protocol.1 Similar preprocessing and analysis pipelines have been applied in many other studies of our research group, using visual stimuli, adjusting the parameters.2,3,4
Functional data were preprocessed using a combination of AFNI9 and FSL’s10 functions. After converting raw functional images to the NIfTI format using dcm2niix17 they were reoriented (FSL’s fslswapdim and fslorient) to correct for marmosets’ posture. The reoriented functional images were preprocessed using procedures for eliminating any outliers (AFNI’s 3dToutcount), despiking (AFNI’s 3dDespike) and time shifting (AFNI’s 3dTshift). We thus registered the images obtained to the base volume (extracted at the half of each run, avoiding the possible initial and final agitation of the animal) using AFNI’s 3dvolreg function. Then, we smoothed all volumes (AFNI’s 3dmerge, FWHM Gaussian kernel of 1 mm) and we applied a bandpass-filter (AFNI’s 3dBandpass, highest frequency of 0.1 Hz and lowest frequency of 0.01 Hz).
The scan timing was then convolved to the BOLD response (AFNI’s 3dDeconvolve) specifying the ‘BLOCK’ convolution and extracting a regressor for each experimental condition (n = 3: Vocalizations, Scrambled Vocalizations and Non-Vocal Sounds) and for each run (n ranging from 6 to 14, depending on the monkey) to be used in the subsequent regression analysis. All the conditions were entered in the model, along with polynomial detrending regressors (n = 5). This regression generated three β-weight maps, corresponding to the three experimental conditions, per animal (n = 9) per run.
In parallel, we generated a T2-weighted template mask for each monkey using their anatomical data (acquired during their first session). Following the reorientation of the anatomical data, we manually skull-stripped the brain, removed the olfactory bulb and binarized the mask obtained using FSLeyes10 In the next step, an average functional image was calculated for each run of each animal and then registered (FSL’s FLIRT function) to the respective T2-weighted image. The transformation matrix thus obtained was subsequently used to register the β-weight maps to the anatomical mask of the animal. We lastly applied the binarized mask to the functional data to extract only the voxels in the brain.
Finally, we registered the anatomical and functional images to the NIH marmoset brain atlas18 via the non-linear registration operated by Advanced Normalization Tools’ (ANTs12) ApplyTransforms function.
The β-weight maps were subsequently averaged for each animal to generate a single map per condition per monkey. These averaged maps were then entered into a mixed-design ANOVA (AFNI’s 3dANOVA2), with Condition (Vocalizations, Scrambled Vocalizations, and Non-Vocal Sounds) as a fixed factor and subject as a random factor (n = 9 marmosets) to account for individual variability. Post hoc comparisons were computed within AFNI’s 3dANOVA2. All the resultant maps were thresholded at p < 0.01 (uncorrected), corresponding to an F-value of 6.22 for the main effect of Condition (all sounds versus baseline) and a T-value of 3.55 for all post hoc paired comparisons.
The small sample size used in non-human primate fMRI studies raises a statistical problem that deserves a brief discussion. It is not the purpose of this manuscript to propose a universal solution to this problem, but rather to provide an example of the statistical methods we employ to report statistical results as reliable as possible. In some of our more recent studies,2,3,4 where the sample included 6–7 animals, we adopted a permutation-based correction at the level of the size of the activation clusters obtained through concatenation of individual runs. While this type of correction takes into account the reduced sample size, it often leads to the loss of small subcortical activation clusters, thereby diminishing the interpretative power of the results.
In the analyses presented here,1 on the other hand, we sought to consider the individual variability present in the data by utilizing a mixed ANOVA model. Similar to the previous case, this choice presents both advantages and disadvantages: on one hand, we can account for subject variability, but on the other hand, an ANOVA with n = 9 (the maximum number of monkeys we can include in our studies currently) poses a statistical challenge, significantly weakening the results of such an analysis. For this reason, the representation of results has been conducted using a rather lenient correction threshold, such as p < 0.01.
Limitations
Our training regimen incorporates a three-week acclimation process to familiarize the animal with the MRI imaging environment. This is intended to reduce stress during experimental sessions and minimize any resulting motor restlessness. Nonetheless, this training does not guarantee the marmoset’s cooperation during MRI scans: the animals are not trained to fixate, keep their eyes open, or carry out any tasks within the scanner.
For experiments involving visual stimuli, this might necessitate a larger number of experimental sessions than initially anticipated, compensating for potential non-compliance from the marmoset. If more specialized training is required (like fixation, saccades, or motor tasks within the scanner), it is essential to plan for a more extensive and prolonged pre-MRI training period.
Troubleshooting
Problem 1
Not all marmosets have the same propensity to enter the body restraint. With some animals it is possible to encounter a considerable resistance, which manifests itself through the use of the upper limbs to counteract the rear pressure of the experimenter or through a positioning of the head that prevents passage through the anterior opening of the body restraint (by lowering the head, for example, the animal can prevent its head post from passing through the opening). With particularly small marmosets (∼300 g), it is also possible that the body restraint provides enough space that the monkey can turn around and try to get out from the rear opening (rarer occasion than the previous two). Problem related to Step 5b.
Potential solution
-
•
Having two experimenters greatly facilitates this step. While one of the two experimenters can use two hands to keep the marmoset in the body restraint and gently push it forward, the second experimenter keeps the neck plate open and tries to attract the animal towards the frontal hole (for example using small pieces of food, marshmallows, or pudding on a stick). If the animal continues to resist, the second experimenter can act accordingly, for example by grasping and moving the upper limbs to reduce the backward thrust or keeping the animal’s head post in a position that allows the head to come out. In the case of small animals, if the attraction of the food is not sufficient, the second experimenter can try to reduce the internal space of the body restraint by inserting something soft but bulky through the front opening, such as one of the thick gloves used for handling marmosets. This will have two effects: reduce the space available to the monkey to turn around and provide something to which the monkey can cling (with the upper limbs or with the teeth). This second effect can represent a further advantage: once the marmoset has grabbed the glove, slowly withdraw it to accompany the rear thrust of the first experimenter.
-
•
In no case wherein the animal is resisting should the rear pressure be increased to force the head out of the front opening. This procedure represents a risk for the marmoset, both because of a possible wrong position inside the body restraint, and because of the possibility that the head post hits an obstacle too violently. Given the thinness of the braincase of these animals, such an impact could have important repercussions on their health.
Problem 2
Keeping the earphones in the correct position can be complicated: due to the weight of the earphones and the connecting cables, in fact, the silicone may not be sufficiently adhesive to remain inside the auricle. This could cause the earphone to come out of the ear, on the one hand nullifying the experiment and on the other hand exposing the animal to the powerful noise of the scanner. Problem related to Step 5k.
Potential solution
-
•
As shown in the Methods video S8, after placing the two earphones it is possible to secure them in the ears using an adhesive elastic band. Pass the band under the marmoset’s chin, sticky side up. Wrap the two ends around the animal’s head being careful to cover the ears, to keep the silicone-wrapped earphones in place. Given the presence of the head post, the two ends cannot cross exactly above the head but must be passed behind the head post. Finally, block the two ends of the elastic band by sticking them under the marmoset’s chin.
Problem 3
In some cases, after performing the localizer and shimming procedure, it is possible to observe a highly distorted image or one with a very low signal. Problem related to Step 8.
Potential solution
-
•
A first, rapid solution can be to repeat the shimming, checking if the new one obtains better results.
-
•
If not, the low/highly distorted signal may be due to incorrect positioning of the two coil housing parts, or eventually a misconnection/bad connection of one or more of the receive channels at the level of the pre-amplifiers. In this case, it is necessary to remove the cradle from the bore, check the entire body restraint and then try again with the localizer and the shimming.
-
•
Finally, a possible reason of bad signal may be the movement of the animal. Even if the body restraint and the head fixation largely limit the possibility of movement, some animals may still present motor agitation, using the upper limb to push the body back and forth, trying to move the head or moving the tail against the connectors. In this case, remove the cradle from the bore, try to immobilize the tail (for example creating a “pocket” with a paper towel, inserting the tail inside it, and then fixing the pocket to the cradle with some adhesive tape) and check if the back plate in the body restraint is thick enough to reduce body movement. Subsequently, push the cradle back into the bore, restart the localizer and the shimming and check the results.
-
•
In relation to the previous point, a potential solution is to extend/resume the training period for animals that exhibit motor agitation during MRI sessions. It is possible that some animals successfully reach the end of training without showing signs of stress or motor agitation, but this situation might not be replicated once the animal is immersed in the actual MRI experimental setting. Differences in environment, the presence of unfamiliar individuals, the noise from the actual scanner, and symptoms induced by the strong magnetic field can cause some animals to regress into a state of stress, fear, and motor agitation. In this case, it is feasible to repeat the third phase of MRI training (outside the scanner) to attempt to reduce such stress. Ideally, this "second" training can take place in a different room from the previous one, possibly in the MRI preparation room where the animal previously experienced heightened stress.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Stefan Everling (severlin@uwo.ca).
Materials availability
This study did not generate new unique reagents.
Acknowledgments
We wish to thank Whitney Froese and Hannah Pettypiece for animal preparation and care and Dr. Kevin Johnston for his assistance during surgical procedures and his valuable comments to the manuscript. Support was provided by the Canadian Institutes of Health Research (FRN 148365, S.E.), the Natural Sciences and Engineering Research Council of Canada (S.E.), and the Canada First Research Excellence Fund to BrainsCAN.
Author contributions
S.E. obtained funding for the study and performed the surgical procedure. K.M.G. and A.L. optimized the fMRI protocol. A.Z., A.D., and A.J. conducted the fMRI experiments and collected and analyzed the data. M.B. optimized the animal preparation before the fMRI session. K.M.G. and P.Z. designed and created the body restraint, the PEEK head post, and all the 3D printed parts described in the manuscript. A.Z., A.D., and S.E. optimized the fMRI analysis pipeline. A.Z. wrote the manuscript. All the authors reviewed the manuscript and gave the final approval.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xpro.2023.102586.
Contributor Information
Alessandro Zanini, Email: zanini.alessandro90@gmail.com.
Stefan Everling, Email: severlin@uwo.ca.
Data and code availability
All the fMRI data (both at individual and group level) and the stimuli described in the study of Jafari and colleagues1 are available on Zenodo: https://zenodo.org/record/7786440#.ZCb1my8r1z8. The CAD files of the body restraint, the PEEK head post and the other 3D printed pieces described in this paper (and in ref.7) are fully available on OSF: http://doi.org/10.17605/OSF.IO/VTQEP. The code for preprocessing and analysis of the fMRI data described in this paper and in ref.1 is available on Zenodo: https://zenodo.org/record/7789270.
References
- 1.Jafari A., Dureux A., Zanini A., Menon R.S., Gilbert K.M., Everling S. A vocalization-processing network in marmosets. Cell Rep. 2023;42 doi: 10.1016/j.celrep.2023.112526. [DOI] [PubMed] [Google Scholar]
- 2.Dureux A., Zanini A., Everling S. Face-Selective Patches in Marmosets Are Involved in Dynamic and Static Facial Expression Processing. J. Neurosci. 2023;43:3477–3494. doi: 10.1523/JNEUROSCI.1484-22.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zanini A., Dureux A., Selvanayagam J., Everling S. Ultra-high field fMRI identifies an action-observation network in the common marmoset. Commun. Biol. 2023;6:553–611. doi: 10.1038/s42003-023-04942-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dureux A., Zanini A., Selvanayagam J., Menon R.S., Everling S. Gaze patterns and brain activations in humans and marmosets in the Frith-Happé theory-of-mind animation task. Elife. 2023;12 doi: 10.7554/eLife.86327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schaeffer D.J., Gilbert K.M., Hori Y., Gati J.S., Menon R.S., Everling S. Integrated radiofrequency array and animal holder design for minimizing head motion during awake marmoset functional magnetic resonance imaging. Neuroimage. 2019;193:126–138. doi: 10.1016/j.neuroimage.2019.03.023. [DOI] [PubMed] [Google Scholar]
- 6.Johnston K.D., Barker K., Schaeffer L., Schaeffer D., Everling S. Methods for chair restraint and training of the common marmoset on oculomotor tasks. J. Neurophysiol. 2018;119:1636–1646. doi: 10.1152/jn.00866.2017. [DOI] [PubMed] [Google Scholar]
- 7.Gilbert K.M., Dureux A., Jafari A., Zanini A., Zeman P., Menon R.S., Everling S. A radiofrequency coil to facilitate task-based fMRI of awake marmosets. J. Neurosci. Methods. 2023;383 doi: 10.1016/j.jneumeth.2022.109737. [DOI] [PubMed] [Google Scholar]
- 8.Ellis D.P.W. Mathworks Inc; 2010. Time-domain Scrambling of Audio Signals in Matlab.http://www.ee.columbia.edu/∼dpwe/resources/matlab/scramble/ [Google Scholar]
- 9.Cox R.W. AFNI: Software for Analysis and Visualization of Functional Magnetic Resonance Neuroimages. Comput. Biomed. Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 10.Smith S.M., Jenkinson M., Woolrich M.W., Beckmann C.F., Behrens T.E.J., Johansen-Berg H., Bannister P.R., De Luca M., Drobnjak I., Flitney D.E., et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 11.Marcus D.S., Harwell J., Olsen T., Hodge M., Glasser M.F., Prior F., Jenkinson M., Laumann T., Curtiss S.W., Van Essen D.C. Informatics and Data Mining Tools and Strategies for the Human Connectome Project. Front. Neuroinform. 2011;5:4. doi: 10.3389/fninf.2011.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Avants B.B., Tustison N.J., Song G. Advanced Normalization Tools (ANTs) Insight J. 2009;2:1–35. [Google Scholar]
- 13.Handler W.B., Bindseil G., Chaddock R., Dalrymple B., Gati J.S., Gilbert K.M., Harris C.T., Klassen M.L., Peterson J., Van Sas F., Chronik B.A. Design and construction of a gradient coil for high resolution marmoset imaging. Biomed. Phys. Eng. Express. 2020;6 doi: 10.1088/2057-1976/ab8d97. [DOI] [PubMed] [Google Scholar]
- 14.Toarmino C.R., Yen C.C.C., Papoti D., Bock N.A., Leopold D.A., Miller C.T., Silva A.C. Functional magnetic resonance imaging of auditory cortical fields in awake marmosets. Neuroimage. 2017;162:86–92. doi: 10.1016/j.neuroimage.2017.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silva A.C., Liu J.V., Hirano Y., Leoni R.F., Merkle H., Mackel J.B., Zhang X.F., Nascimento G.C., Stefanovic B. In: Magnetic Resonance Neuroimaging: Methods and Protocols. Modo M., Bulte J.W.M., editors. Humana Press; 2011. Longitudinal functional magnetic resonance imaging in animal models; pp. 281–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paxinos G., Watson C., Petrides M., Rosa M.G.P., Tokuno H. Elsevier Academic Press; 2012. The Marmoset Brain in Stereotaxic Coordinates. [Google Scholar]
- 17.Li X., Morgan P.S., Ashburner J., Smith J., Rorden C. The first step for neuroimaging data analysis: DICOM to NIfTI conversion. J. Neurosci. Methods. 2016;264:47–56. doi: 10.1016/j.jneumeth.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Liu C., Ye F.Q., Yen C.C.-C., Newman J.D., Glen D., Leopold D.A., Silva A.C. A digital 3D atlas of the marmoset brain based on multi-modal MRI. Neuroimage. 2018;169:106–116. doi: 10.1016/j.neuroimage.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the fMRI data (both at individual and group level) and the stimuli described in the study of Jafari and colleagues1 are available on Zenodo: https://zenodo.org/record/7786440#.ZCb1my8r1z8. The CAD files of the body restraint, the PEEK head post and the other 3D printed pieces described in this paper (and in ref.7) are fully available on OSF: http://doi.org/10.17605/OSF.IO/VTQEP. The code for preprocessing and analysis of the fMRI data described in this paper and in ref.1 is available on Zenodo: https://zenodo.org/record/7789270.