Abstract
Background
Acute postoperative pain is still an issue in patients undergoing abdominal surgery. Postoperative pain and side effects of analgesic treatment, in particular those of opioids, need to be minimized. Opioid‐sparing analgesics, possibly including dexmedetomidine, seem a promising avenue by which to improve postoperative outcomes.
Objectives
Our primary aim was to determine the analgesic efficacy and opioid‐sparing effect of perioperative dexmedetomidine for acute pain after abdominal surgery in adults.
Secondary aims were to establish effects of dexmedetomidine on postoperative nausea and vomiting (PONV), gastrointestinal function and mobilization, together with the side effect profile of dexmedetomidine.
Search methods
We searched the following databases: Cochrane Central Register of Controlled Trials, MEDLINE, EMBASE, Institute for Scientific Information (ISI), Web of Science and Cumulative Index to Nursing and Allied Health Literature (CINAHL), and reference lists of articles to May 2014. We searched the Science Citation Index, ClinicalTrials.gov and Current Controlled Trials, and we contacted pharmaceutical companies to identify unpublished and ongoing studies. We applied no language restrictions. We reran the search in May 2015 and found nine studies of interest. We will deal with the studies of interest when we update the review.
Selection criteria
We included randomized, controlled trials of perioperative dexmedetomidine versus placebo or other drug during abdominal surgery in adults. Trials included one of the following outcomes: amount of 'rescue' opioid, postoperative pain, time to 'rescue' analgesia, participants requiring 'rescue' analgesia, postoperative sedation, PONV, time to first passage of flatus and stool or time to first out‐of‐bed mobilization.
Data collection and analysis
Two review authors independently screened the titles and abstracts for eligibility. We retrieved full trial reports if necessary, and we extracted relevant data from the included studies using a data collection form and assessed risk of bias. We resolved disagreements by discussion with the third review author. We sought additional information of relevance for risk of bias assessment or extraction of data by contacting study authors or, if necessary, co‐authors from present or former studies.
Main results
Our systematic review included seven studies with a total of 492 participants. We included 422 participants in our analysis. Thirteen studies are awaiting classification. For the comparison dexmedetomidine versus placebo (six studies, 402 participants), most studies found a reduction in 'rescue' opioid consumption in the first 24 hours after surgery, together with in general no clinically important differences in postoperative pain (visual analogue scale (VAS) 0 to 100 mm, where 0 = no pain and 100 = worst imaginable pain) in the first 24 hours after surgery ‐ except for one study (80 participants) with a reduction in VAS pain at two hours after surgery in favour of dexmedetomidine, with a mean difference of ‐30.00 mm (95% confidence interval (CI) ‐38.25 to ‐21.75). As the result of substantial heterogeneity, pooling of data in statistical meta‐analyses was not appropriate. The quality of evidence was very low for our primary outcomes because of imprecision of results and risk of bias. Regarding our secondary aims, evidence was too scant in general to allow robust conclusions, or the estimates too imprecise or of poor methodological quality. Regarding adverse effects, low quality data (one study, 80 participants) suggest that the proportion of participants with hypotension requiring intervention was slightly higher in the high‐dose dexmedetomidine group with a risk ratio of 2.50 (95% CI 0.94 to 6.66), but lower doses of dexmedetomidine led to no differences compared with control. Evidence for the comparison dexmedetomidine versus fentanyl was insufficient to permit robust conclusions (one study, 20 participants).
Authors' conclusions
Dexmedetomidine, when administered perioperatively for acute pain after abdominal surgery in adults, seemed to have some opioid‐sparing effect together with in general no important differences in postoperative pain when compared with placebo. However the quality of the evidence was very low as the result of imprecision, methodological limitations and substantial heterogeneity among the seven included studies. The clinical importance for patients is uncertain, in as much as the influence of dexmedetomidine on patient‐important outcomes such as gastrointestinal function, mobilization and adverse effects could not be satisfactorily determined. All included studies were relatively small, and publication bias could not be ruled out. Applicability of evidence was limited to middle‐aged participants who were relatively free of co‐morbidity and were undergoing elective abdominal surgery. A potential bias was a considerable quantity of unobtainable data from studies with mixed surgery. To detect and investigate patient‐important outcomes, larger studies with longer periods of follow‐up are needed.
Plain language summary
Dexmedetomidine for prevention of acute pain after abdominal surgery in adults
Background and review question
Acute pain after surgery is a problem for patients undergoing abdominal surgery. In addition to postoperative pain, the side effects of treatment with pain killers, in particular those of opioids (drugs resembling morphine), need to be reduced. Dexmedetomidine is an opioid sparing drug (reduces the need for opioids). We reviewed the evidence about the effectiveness of dexmedetomidine in reducing the need for opioids and in preventing acute pain after abdominal surgery in adults. We wanted to discover how safe dexmedetomidine was and whether it was effective in preventing some of the known side effects of opioids, such as nausea and vomiting, reduced bowel function and delayed mobilization (getting up and moving around) after abdominal surgery.
Study characteristics
Evidence is current to May 2014. We included seven studies with 492 participants from five different countries and included 422 participants in our analysis. Most participants were middle‐aged. Participants had almost no diseases other than their reason for having surgery. The type of surgery was planned abdominal surgery. Three of the seven studies looked only at obesity surgery. Participants received dexmedetomidine right before or during their abdominal surgery. Six studies compared dexmedetomidine with no treatment, and one small study compared dexmedetomidine with fentanyl (a strong opioid).
We reran the search in May 2015 and found nine studies of interest, which we will discuss when we update the review. In total, 13 studies are awaiting classification.
Key results and quality of the evidence
Most of the studies that compared dexmedetomidine with no treatment found that dexmedetomidine reduced the need for opioids for treating pain for 24 hours after surgery. During the same period, no important differences in pain were noted, except one study (80 participants) showed a reduction in intensity of pain at two hours after surgery with dexmedetomidine. The quality of the evidence was very low because the results were not similar across studies, and because some studies were poorly conducted. The influence of dexmedetomidine on postoperative nausea and vomiting could not be determined because results were not similar across studies. No conclusion could be made for bowel function and mobilization and side effects such as postoperative sedation, as data were insufficient. One study with 80 participants reported a higher rate of low blood pressure ('low' meaning that medication was required) for participants receiving a high dose of dexmedetomidine compared with no treatment, but for lower doses of dexmedetomidine, they noted no differences compared with no treatment.
For the comparison dexmedetomidine versus fentanyl, data were insufficient to allow conclusions (only one small study).
Conclusion
Dexmedetomidine ‐ compared with no treatment ‐ seemed to reduce the need for opioids without worsening the experience of postoperative pain after abdominal surgery in adults. However, the quality of evidence was very low because studies were poorly conducted and because results were not similar across studies. The importance of these findings for patients was also uncertain because the influence of dexmedetomidine on bowel function, mobilization and adverse effects could not be properly determined.The seven included studies were small, so side effects associated with use of dexmedetomidine may be greater than this review reported. In addition, we could not obtain relevant data from several studies because investigators mixed abdominal surgery with other types of surgery.
Summary of findings
for the main comparison.
| Dexmedetomidine for postoperative pain | ||||
|
Patient or population: adults having abdominal surgery Setting: hospital Intervention: perioperative dexmedetomidine | ||||
| Comparison: dexmedetomidine vs placebo | ||||
| Outcome | Effecta | Number of participants (number of studies) | Quality of evidence (GRADE)* | Comments |
| Amount of 'rescue' opioid (intravenous morphine equivalents, mg) 24 hours after surgery (MD, 95% CI) | Three of four studies found a reduction in 'rescue' opioid consumption (Bakhamees 2007; Mohamed 2012; Tufanogullari 2008). One study showed reduced need for non‐opioid analgesia in the dexmedetomidine group and no difference in consumption of 'rescue' opioid (Park 2012, 42 participants) | 259 (4 studies) | ⊕⊝⊝⊝ very low | Downgraded 3 levels because of serious risk of bias (Mohamed 2012; Park 2012; Tufanogullari 2008), serious imprecision of results (Park 2012; Tufanogullari 2008) and serious inconsistency arising from heterogeneity in effect estimates |
| Postoperative pain (VAS 0 to 100 mm, visual analogue scale from 0 = no pain to 100 = worst imaginable pain) 24 hours after surgery (MD, 95% CI) | No clinically important difference in VAS postoperative pain | 259 (4 studies) | ⊕⊝⊝⊝ very low | Downgraded 3 levels because of serious risk of bias (Mohamed 2012; Park 2012; Tufanogullari 2008), serious imprecision and serious inconsistency arising from heterogeneity in amount of 'rescue' analgesia between studies |
| Postoperative sedation 12 hours after surgery (units of RSS, Ramsay Sedation Scale from 1 = Anxious or agitated or restless to 6 = Unresponsive) (MD, 95% CI) | Quantity of data was too small to allow a robust conclusion. The only study reporting data found an increased level of sedation with dexmedetomidine, with a mean difference in RSS 1 to 6 of 1.60 units (95% CI 1.49 to 1.71) (Xiao 2013) | 80 (1 study) | ⊕⊝⊝⊝ very low | Downgraded 3 levels because of serious risk of bias and serious imprecision (small quantity of data) (Xiao 2013) |
| Postoperative nausea and vomiting (PONV) (RR, 95% CI) | One study found reduced risk of PONV with dexmedetomidine (RR 0.54, 95% CI 0.33 to 0.87; Tufanogullari 2008, 77 participants) and at the same time reduced need for antiemetics. Two other studies found risk ratios of PONV close to favouring neither dexmedetomidine nor placebo (RR 0.67, 95% CI 0.12 to 3.78 for Bakhamees 2007, 80 participants) (RR 0.50, 95% CI 0.17 to 1.48 for Mohamed 2012, 60 participants) | 217 (3 studies) | ⊕⊕⊝⊝ low |
Downgraded 2 levels because of serious inconsistency in outcome definition (PONV separated in nausea and vomiting) (Bakhamees 2007; Mohamed 2012; Tufanogullari 2008) and risk of bias (Bakhamees 2007; Mohamed 2012) |
| Time to first passage of flatus (hours, MD, 95% CI) | Quantity of data was too small to allow a robust conclusion. The only study reporting data found no difference in time to first passage of flatus (Tufanogullari 2008) | 77 (1 study) | ⊕⊕⊝⊝ low |
Downgraded 2 levels because of very serious imprecision (small quantity of data) |
| Time to first passage of stool (hours, MD, 95% CI) | NR | NR | ||
| Time to first out‐of‐bed mobilization (hours, MD, 95% CI) | Quantity of data was too small to allow a robust conclusion. The only study reporting data found no difference in time to first out‐of‐bed mobilization (Tufanogullari 2008) | 77 (1 study) | ⊕⊕⊝⊝ low |
Downgraded 2 levels because of very serious imprecision (small quantity of data) |
| MD: mean difference; CI: confidence interval; RR: risk ratio; NR: no data reported | ||||
| *GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||
aPerforming meta‐analyses was not appropriate for any outcome. All studies had a relatively small number of participants and substantial heterogeneity for three main reasons:
1. Methodological limitations in several studies (high or unclear risk of bias)
2. Clinical variation with great difference in amount of 'rescue' analgesia, type of surgery, body mass index, route and timing of administration of dexmedetomidine and anaesthetic agents, etc
3. Statistical variation with relatively great imprecision in results and a high I2 statistic for several outcomes
Background
Description of the condition
Acute postoperative pain is still an issue in patients undergoing abdominal surgery. Inefficient relief of pain may be associated with reduced mobility, postoperative complications and prolongation of hospital stay (Kehlet 2003). Although different analgesic agents and techniques are available, opioids remain one of the cornerstones of postoperative pain treatment because of their excellent analgesic properties. Nevertheless, opioids have worrisome side effects such as nausea, vomiting, gastrointestinal dysfunction, drowsiness, urinary retention and respiratory depression. These adverse effects may sometimes outweigh analgesic benefits, as they may impair postoperative rehabilitation as well (Bonnet 2007). The gastrointestinal side effects of opioids are particularly undesirable after abdominal surgery, which in itself is associated with paralytic ileus. It has been recommended to use opioids after abdominal surgery only when non‐opioid drugs provide insufficient analgesia (Kehlet 2003). Consequently, there is a need to develop and extend the use of non‐opioid analgesia for acute pain after abdominal surgery.
Description of the intervention
Dexmedetomidine is an alpha‐2 adrenoceptor agonist with sedative, analgesic, sympatholytic and anxiolytic properties. Indications for use are presently limited to sedation in intensive care units (ICUs) (Dexdor product information; Precedex prescribing information) and sedation in surgical patients who are not intubated (Precedex prescribing information). A recent clinical practice guideline suggests dexmedetomidine for the treatment of delirium in the ICU (Barr 2013). Dexmedetomidine is presently licensed for intravenous use only, but other modes of administration are being explored, including intramuscular, epidural, intra‐articular, buccal and intranasal routes (Chan 2010). The most common adverse effects of dexmedetomidine are hypotension, bradycardia, hypertension and nausea; less common adverse effects include atrial fibrillation, fever and dry mouth, and cases of sinus arrest have been reported (Dexdor product information; Precedex prescribing information). Infusion of dexmedetomidine exceeding 24 hours is not recommended because of risk of agitation and respiratory failure (Precedex prescribing information); this precaution is not stated in Dexdor product information. The same drug dexmedetomidine is sold as Precedex and Dexdor under two different regulatory authorities.
How the intervention might work
The analgesic properties of dexmedetomidine probably involve both peripheral and central mechanisms. Centrally, the antinociceptive effect seems to be related to stimulation of alpha‐2 adrenoceptors located both at the spinal level in neurons of the dorsal horn and at a supraspinal level in the locus coeruleus. The peripheral analgesic mechanism is not entirely elucidated (Chan 2010). Dexmedetomidine is eight times more selective of alpha‐2 adrenoceptors than clonidine; thus it is expected to be a more effective analgesic agent (Chan 2010).
Several trials have demonstrated a significant opioid‐sparing effect of dexmedetomidine (Arain 2004; Gurbet 2006; Lin 2009; Unlugenc 2005; Vandermeulen 2006). A great benefit is that dexmedetomidine does not seem to compromise respiratory function (Mantz 2011; Villela 2003). Other possible benefits include neuroprotection (attenuation of delirium) and cardioprotection (prevention of myocardiac ischaemia), although these have not been convincingly demonstrated (Biccard 2008; Chrysostomou 2008; Mantz 2011; Wijeysundera 2009).
Why it is important to do this review
To improve postoperative outcomes after abdominal surgery, both postoperative pain and side effects of analgesic treatment, in particular those of opioids, must be minimized. The physiological mechanisms of postoperative pain operate at several different levels; thus multi‐modal or balanced analgesia is generally recommended (Kehlet 2003). In abdominal surgery, the opioid side effects are particularly undesirable. As side effects increase at higher doses (Bonnet 2007; Marret 2005), opioid‐sparing analgesics seem a promising avenue by which postoperative outcomes can be improved. In this context, it is appropriate to examine critically the risks and benefits of dexmedetomidine.
As a result of its opioid‐sparing effect, it is plausible that patients treated with dexmedetomidine after abdominal surgery will experience better gastrointestinal function (less nausea and vomiting and shorter duration of paralytic ileus). Thus, there is reason to believe that the use of dexmedetomidine is associated with fewer postoperative complications and facilitates recovery after abdominal surgery. This assumption needs to be investigated, as it has been shown that greater analgesic efficacy does not automatically translate into improved clinical postoperative outcomes such as recovery of bowel function, active mobilization and fewer organ‐related complications (Liu 2007; White 2010). Moreover, studies differ as to whether postoperative recovery is facilitated by the use of dexmedetomidine (Tan 2010; Unlugenc 2005).
Another question that needs to be addressed is whether the analgesic efficacy of dexmedetomidine comes at the cost of problematic side effects, in particular, postoperative sedation and cardiovascular side effects including bradycardia and hypotension (Arain 2004; Biccard 2008; Lin 2009; Tan 2010). With regards to postoperative nausea and vomiting (PONV), some studies show that dexmedetomidine has a lower incidence of nausea compared with opioids (Lin 2009). Other studies show that dexmedetomidine has no effect on the incidence of opioid‐induced bowel dysfunction despite its opioid‐sparing properties (Vandermeulen 2006).
Therefore, this systematic review is needed in order to clarify the analgesic properties of dexmedetomidine when used perioperatively in abdominal surgery, as well as to establish the harms and benefits associated with its use.
Objectives
Our primary aim was to determine the analgesic efficacy and opioid‐sparing effect of perioperative dexmedetomidine for acute pain after abdominal surgery in adults.
Secondary aims were to establish effects of dexmedetomidine on PONV, gastrointestinal function and mobilization, together with the side effect profile of dexmedetomidine.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) evaluating the effect of dexmedetomidine for acute pain after abdominal surgery in adults, irrespective of language and publication status. We would have included cluster‐randomized trials and factorial trials (in a factorial trial, at least two intervention comparisons are carried out simultaneously), had we identified any.
We excluded cross‐over trials, quasi‐randomized trials and all non‐randomized trials.
Types of participants
We included adult participants undergoing all types of abdominal surgery, including both open and laparoscopic procedures. We included general and regional forms of anaesthesia. We defined abdominal surgery as surgery to intra‐abdominal organs, excluding gynaecological, urological, vascular and superficial surgery (such as hernia repair).
Types of interventions
We compared perioperative (preoperative, intraoperative or postoperative) administration of dexmedetomidine with other treatments or placebo (with 'rescue' medication). We included all modes of administration and all variations of dosage, frequency and duration.
We included interventions combining dexmedetomidine with another treatment if that same treatment, without dexmedetomidine, was given to the control group. We also included interventions combining dexmedetomidine with another treatment if the design of the trial was factorial, and if we did not suspect any interaction between treatments.
Types of outcome measures
Primary outcomes
The opioid‐sparing effect of dexmedetomidine ‐ measured by amount of 'rescue' opioid, administered via any route, at three, six, 12 and 24 hours after end of surgery.
The analgesic efficacy of dexmedetomidine ‐ measured at rest and on movement, as defined by study authors, by visual analogue scale (VAS) 0 to 100 mm, where 0 mm corresponds to no pain and 100 mm corresponds to worst imaginable pain. Use of a VAS scale 0 to 10 cm was converted to VAS 0 to 100 mm. We regarded any use of verbal or numerical rating scales (NRSs) from 0 to 10 as convertible with VAS. We selected the measuring time points of three, six, 12 and 24 hours after end of surgery.
Secondary outcomes
Time to first request of 'rescue' analgesia.
Proportion of participants needing 'rescue' analgesia.
Postoperative sedation ‐ assessed by clinical measures at three, six and 12 hours after end of surgery. By 'clinical measures', we understood values estimated by observer or participant, such as the Ramsey Sedation Scale (RSS), and not by use of technology, such as the bispectral index (BIS).
Proportion of participants with PONV until 24 hours after end of surgery, or proportion of participants treated with antiemetics.
Time to first passage of flatus after end of surgery or proportion of participants with delay to first passage of flatus.
Time to first passage of stool after end of surgery or proportion of participants with delay to first passage of stool.
Time to first out‐of‐bed mobilization after end of surgery or proportion of participants with delay to first out‐of‐bed mobilization.
Post‐interventional complications or adverse effects, particularly hypotension, bradycardia, delirium and respiratory failure, reported as a proportion of participants.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases: Cochrane Central Register of Controlled Trials (CENTRAL; 2014, Issue 5; see Appendix 1); MEDLINE, Ovid SP (1956 to May 2014; see Appendix 2); EMBASE, Ovid SP (1982 to May 2014; see Appendix 3); Institute for Scientific Information (ISI) Web of Science (1950 to May 2014; see Appendix 4) and Cumulative Index to Nursing and Allied Health Literature (CINAHL) via EBSCO host (1980 to May 2014; see Appendix 5). When searching the databases, we used both subject headings and free‐text terms. We combined our subject search terms with the Cochrane highly sensitive search strategy for identifying RCTs, as suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We adapted our MEDLINE search strategy when searching all other databases.
We searched the references in accepted studies for additional eligible trials.
We reran the search in May 2015.
We applied no language restrictions.
Searching other resources
We searched the references in accepted studies for additional eligible trials.
We searched the Science Citation Index, ClinicalTrials.gov and Current Controlled Trials in August 2014 to identify additional published, unpublished and ongoing studies.
Furthermore, we contacted the medical companies responsible for marketing of dexmedetomidine, to ask about additional and unpublished research.
Data collection and analysis
Selection of studies
Two review authors (LJL, HKN) independently examined the titles and abstracts obtained by the above searches. We excluded trials that did not meet all of the eligibility criteria referred to on the data collection form (Appendix 6). If a decision could not be made on the basis of the abstract alone, we retrieved the full trial report. We documented the reason for exclusion of trials when the reason for exclusion was not obvious.
We resolved disagreements between the two review authors by consulting with a third review author (AMM).
If a trial report provided insufficient information for a decision on inclusion, we contacted the first author of the trial.
We (LJL, HKN) were not blinded to reference details during the selection process.
Data extraction and management
Two review authors (LJL, HKN) went through the full text of all included trials. We independently completed the data collection form (Appendix 6) in the process of extracting data.
Both review authors (LJL, HKN) performed a pilot test of the data collection form.
We resolved disagreements by discussion, and if they remained unresolved, by consulting with a third review author (AMM). When disagreements remained, we contacted the first author of the relevant trial to seek further information. If we could not resolve a disagreement, we reported this in our review.
Assessment of risk of bias in included studies
Two review authors (LJL, HKN) independently assessed the risk of bias for each eligible trial. We resolved disagreements by discussion with a third review author (AMM).
We performed the assessment of risk of bias as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We assessed the risk of bias by evaluating the following seven domains in each trial: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other bias. On the basis of the description of the study's approach to each domain (including relevant quotes and our comments), we made a judgement of high, low or unclear risk of bias for each domain, using the specific criteria recommended by Higgins 2011.
We originally planned to include studies in our meta‐analysis regardless of our assessment of risk of bias for an outcome as high, low or unclear. Subsequently, and if data were sufficient, we planned to perform sensitivity analyses, first by excluding studies with high risk of bias for the outcome in question, and second by excluding studies with high and unclear risk of bias. However, no meta‐analyses were appropriate.
Measures of treatment effect
For dichotomous outcomes, we chose risk ratio (RR) as the preferred effect measure. If relevant, we planned to calculate the number needed to treat for an additional beneficial outcome (NNTB) and the number needed to treat for an additional harmful outcome (NNTH), although we did not intend to incorporate these into the meta‐analyses.
For continuous outcomes, we calculated the difference in means or the mean difference (MD) when measurement scales were similar. If measurement scales were not similar, we planned to use the standardized mean difference (SMD).
We treated ordinal outcomes and measurement scales as continuous data.
Unit of analysis issues
To avoid making a unit of analysis error, we would be particularly cautious when facing a trial that did not follow a 'standard' design, meaning one measurement for each outcome for each participant in a double‐arm trial. The two following cases need to be mentioned specifically.
For any study with multiple treatment arms, we tried to combine groups to create a single comparison. But if the combination of groups was not meaningful, we judged the relevance of all treatment groups for our review question. If one or several treatment arms were not relevant, we excluded these from the analysis, although we mentioned them under Characteristics of included studies and evaluated whether the exclusion introduced any risk of bias. If all treatment arms were relevant to our review question, we planned to perform a multiple treatments meta‐analysis (MTM).
For any study that provided multiple observations for the same outcome, we performed a separate analysis for each relevant observation, expecting this to be the case for several of our selected outcomes: amount of 'rescue' opioid (at three, six, 12 and 24 hours after surgery), postoperative pain at rest and on movement (at three, six, 12 and 24 hours after surgery) and postoperative sedation (at three, six and 12 hours after surgery).
Dealing with missing data
For any type of missing data, we contacted the first author of the relevant trial to ask for additional information. If the contact information was not directly available, we tried to retrieve an email address or a postal address by searching the Internet, or by contacting co‐authors from present or former studies. Some attempted correspondence may not have reached the addressee.
When we encountered missing data, we planned to perform an intention‐to‐treat (ITT) analysis if possible. If an ITT analysis was not possible, we based our analysis on available data and discussed risk of bias and the potential impact of missing data. In any meta‐analyses, we planned to perform a sensitivity analysis for missing data concerning best‐case and worst‐case scenarios.
Assessment of heterogeneity
We considered heterogeneity arising from clinical diversity (related to participants, interventions and outcomes) and from methodological diversity (related to risk of bias) to be present a priori. We quantified statistical heterogeneity by using the I2 statistic, which reflects the percentage of variability that is due to heterogeneity rather than to random error.
We planned to perform meta‐analyses. A meta‐analysis would be appropriate, though, only if variation in results was not considerable, as judged by clinical and methodological measures and by the statistical measure of heterogeneity, the I2 statistic, which ideally but not necessarily should be below 75% (Higgins 2011). Additionally, a meta‐analysis would be appropriate only if the amount of information was sufficient (size and number of trials) (Higgins 2011).
Assessment of reporting biases
We planned to detect publication bias (forming part of small‐study effects) by creating funnel plots for our primary outcomes. As fewer than 10 studies were included in this review (seven included), we were not able to create a funnel plot. (See Differences between protocol and review for the methods we will apply if future updates of this review include enough studies to permit use of a funnel plot.)
Data synthesis
As mentioned in the Assessment of heterogeneity section, we planned to perform a meta‐analysis if heterogeneity was not considerable. However, because of either a small number of studies or considerable heterogeneity, we performed no meta‐analyses. (See Differences between protocol and review for the methods that will be applied if future updates of this review permit meta‐analyses.)
Among our selected outcomes, we did not expect post‐interventional complications and adverse effects to be suitable for a meta‐analysis or for inclusion in the 'Summary of findings' table. Instead, we intended to prepare a narrative report.
Subgroup analysis and investigation of heterogeneity
We were not able to perform subgroup analyses, as no meta‐analyses were appropriate. See Differences between protocol and review.
Sensitivity analysis
We were not able to perform sensitivity analyses, as no meta‐analyses were appropriate. See Differences between protocol and review.
Summary of findings
We used the principles of the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) system (Guyatt 2008) in our review to assess the quality of the body of evidence associated with specific outcomes (amount of 'rescue' opioid, postoperative pain, postoperative sedation, PONV, time to first passage of flatus, time to first passage of stool, time to first out‐of‐bed mobilization) and to construct a 'Summary of findings' table, if possible using GRADE software. The GRADE approach appraises the quality of a body of evidence according to the extent to which confidence indicates that an estimate of effect or association reflects the item being assessed. The quality of a body of evidence considers within‐study risk of bias (methodological quality), directness of the evidence, heterogeneity of the data, precision of effect estimates and risk of publication bias.
Results
Description of studies
Results of the search
Our systematic search of databases revealed 2137 records, which amounted to 1883 records when duplicates were removed. By searching other resources, we identified one potentially relevant record from a reference list in the article by Yacout 2012 (Lawrence 1997), and we found six ongoing studies by searching ClinicalTrials.gov, Science Citation Index and Current Controlled Trials (Awad 2014; Jung 2014; Kim 2014; Wai 2014; Yoo 2014; Zeeni 2014). Of the 1890 potentially relevant records, we excluded 1783 records as not pertinent upon screening titles and abstracts. We screened 101 full‐text articles and excluded 49 for obvious reasons. Of the 58 remaining studies, we excluded 32 for specific reasons mentioned for each study under Characteristics of excluded studies and listed these in groups in the study flow diagram (Figure 1). Of the remaining 26 studies, we could not classify 13 because we needed additional information from study authors (Altindis 2008; Anvaroglu 2008; Arain 2004; Bicer 2006; Ceballos 2011; Kilicaslan 2006; Kordan 2006; Mizrak 2010; Scheinin 1992; Subasi 2012; Unlugenc 2005; Yacout 2012; Yektas 2011; see Characteristics of studies awaiting classification), and we found that six were ongoing studies (see Characteristics of ongoing studies). This process resulted in inclusion of seven studies in the narrative synthesis and revealed none for inclusion in a quantitative synthesis (meta‐analysis).
1.
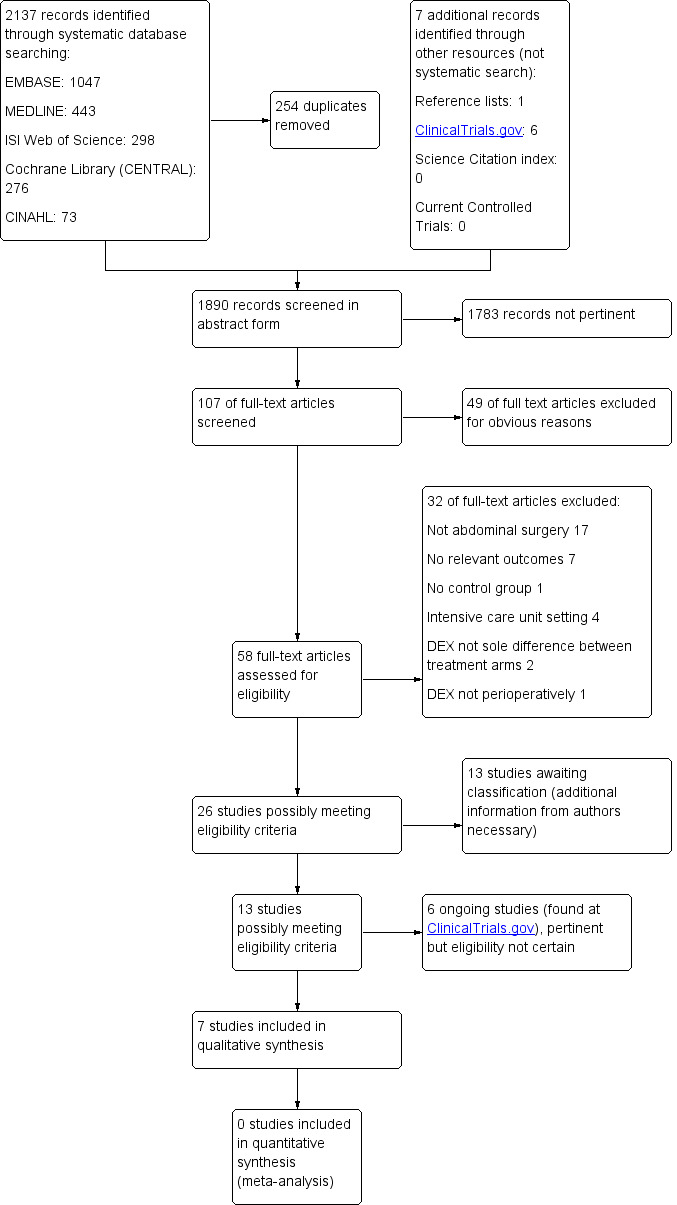
Study flow diagram
We reran the search in May 2015. We found 250 records and identified nine new studies of interest. We listed the nine studies of interest under Studies awaiting classification and in Table 2, and we will incorporate them into formal review findings during the review update.
1. Studies awaiting classification from May 2015 search.
| Cho 2014 | Perioperative infusion of lidocaine vs dexmedetomidine; effect on reduced consumption of postoperative analgesics after laparoscopic cholecystectomy |
| Ibacache 2014 | Effect of dexmedetomidine on postoperative glucose levels and insulin secretion in obese patients with impaired glucose tolerance |
| Jun 2014 | Laparoscopic appendectomy under spinal anaesthesia with dexmedetomidine infusion |
| Kim JM 2014 | Randomized comparative study on the effects of epidural dexmedetomidine on heart rate variability during general anaesthesia in participants undergoing gastrectomy |
| Le Guen 2014 | Dexmedetomidine reduces propofol and remifentanil requirements during bispectral index‐guided closed‐loop anaesthesia: a double‐blind, placebo‐controlled trial |
| Naja 2014 | Effect of clonidine vs dexmedetomidine on pain control after laparoscopic gastric sleeve: a prospective, randomized, double‐blinded study |
| Singh 2014 | Effect of dexmedetomidine on haemodynamics, fentanyl requirement and recovery profile in patients with laparoscopic cholecystectomy |
| Wang 2015 | Effects of dexmedetomidine on patients undergoing radical gastrectomy |
| Ziemann‐Gimmel 2014 | Opioid‐free total intravenous anaesthesia reduces postoperative nausea and vomiting in bariatric surgery beyond triple prophylaxis |
We reran the search in May 2015. These potential new studies of interest published between May 2014 and May 2015 will be incorporated into formal review findings during the review update
Included studies
We included seven studies in this review (Bakhamees 2007; Feld 2006; Khanduja 2013; Mohamed 2012; Park 2012; Tufanogullari 2008; Xiao 2013). The total number of participants was 492, and sample sizes ranged from 20 to 120 participants. After we excluded some intervention arms, our analysis included 422 participants,and sample sizes from the seven studies ranged from 20 to 80 participants.
Design
All seven studies were randomized controlled trials with parallel groups. Three trials had multiple arms: One study comprised three arms with different dosages of dexmedetomidine compared with a fourth placebo arm (Tufanogullari 2008); for our statistical analysis, we pooled the three dexmedetomidine groups. Another study compared dexmedetomidine with dexmedetomidine‐fentanyl and control (Mohamed 2012). To avoid a potential synergy of different medical interventions, we chose to exclude the dexmedetomidine‐fentanyl arm. The third study (Xiao 2013) compared a dexmedetomidine plus high‐dose remifentanil group versus a placebo plus high‐dose remifentanil group versus a placebo plus low‐dose remifentanil group. For this study, we excluded the latter arm to meet our eligibility criteria, with dexmedetomidine as the sole difference between groups. We included no factorial trials and no cross‐over trials. All studies were single‐centre studies. See Characteristics of included studies and Table 3 for additional details. These tables describe the actual design of the study, regardless of our decisions to exclude or pool intervention groups.
2. Baseline data and overview of characteristics of included studies.
| Study (n), country | Groups (n) | Route and mode of administration | Participants | Type (n) and duration of surgery (minutes) | 'Rescue' analgesia |
Other relevant medication 1. Premedication 2. During surgery |
||||
| age (years) | weight (kg) | height (cm) | gender M/F (n) | ASA (n) | ||||||
|
Bakhamees 2007 (80) Egypt |
DEX (40) | Intravenous bolus (0.8 μg/kg) plus infusion (0.4 μg/kg/h) | 30 ± 6 | 123 ± 27 | 169 ± 10 | 16/14c | II: 26 III: 14 |
Laparoscopic gastric bypass 157 ± 29 |
PCA morphine |
1.Dexamethazone 8 mg, midazolam 3 mg 2. Propofol 1447 ± 310 mg, fentanyl 199.4 ± 44.6 μg |
| Placebo (40) | Same volume and rate | 29 ± 8 | 119 ± 21 | 168 ± 8 | 18/12c | II: 24 III: 16 |
Laparoscopic gastric bypass 155 ± 27 |
PCA morphine |
1.Dexamethazone 8 mg, midazolam 3 mg 2. Propofol 2162 ± 454 mg, fentanyl 362.2 ± 57.2 μg |
|
|
Feld 2006 (20) USA |
DEX (10) | Intravenous bolus (0.5 μg/kg) plus infusion (0.4 μg/kg/h) | 40 ± 8 | 175 ± 49a | 152 ± 13a | 4/6 | II: 6 III: 4 |
Open gastric bypass 234 ± 28 |
PCA morphine |
1. Midazolam 2 mg 2. Thiopental 2.1 ± 0.5 mg/kg, less desflurane than control group |
| Fentanyl (10) | Intravenous bolus (0.5 μg/kg) plus infusion (0.5 μg/kg/h) | 39 ± 6 | 159 ± 27a | 152 ± 8a | 3/7 | II: 6 III: 4 |
Open gastric bypass 229 ± 30 |
PCA morphine |
1. Midazolam 2 mg 2. Thiopental 3.1 ± 0.6 mg/kg, more desflurane than DEX group |
|
|
Khanduja 2013 (60) India |
DEX (30) | Intravenous infusion (initiated at 0.5 and augmented to 0.6 μg/kg/h) | 42.2 ± 12.1 | 57 ± 7.03 | NR | 6/24 | I‐II?d | Laparoscopic cholecystectomy NR |
Pentazocineh |
1. Esomeprazole 40 mg 2. End‐inspiratory isoflurane 0.6%, pentazocine 17.9 ± 4.13 mgk |
| Placebo (30) | Same volume and rate | 40.4 ± 11.1 | 56.6 ± 7.45 | NR | 6/24 | I‐II? | Laparoscopic cholecystectomy NR |
Pentazocine |
1. Esomeprazole 40 mg 2. End‐inspiratory isoflurane 1.2%, pentazocine 29.4 ± 4.27 mg |
|
|
Mohamed 2012 (90) Egypt |
DEX (30) | Intrathecal bolus dexmedetomidine 5 µg plus bupivacaine 0.5% 10 mg |
45 ± 2 | 72.8 ± 1.7 | 164 ± 1,5 | 12/18 | I: 27e II: 3 |
Major abdominal cancerg 188 ± 53 |
IV tramadol if VAS pain ≥ 3 |
1. Oral diazepam 5 mg 2. Anaesthesia not described |
| DEX + fentanyl (30)b | Intrathecal bolus dexmedetomidine 5 µg plus fentanyl 25 µg plus bupivacaine 0.5% 10 mg |
44 ± 2 | 73.0 ± 1.7 | 163 ± 1.4 | 8/22 | I: 25 II: 5 |
Major abdominal cancer 190 ± 62 |
Same as above | Same as above | |
| Placebo (30) | Intrathecal bolus of bupivacaine 0.5% 10 mg |
44 ± 2 | 72.8 ± 0.7 | 163.7 ± 1.3 | 10/20 | I: 26 II: 4 |
Major abdominal cancer 173 ± 62 |
Same as above |
1. Oral diazepam 5 mg 2. Anaesthesia not described |
|
|
Park 2012 (42) Korea |
DEX (21) | Intravenous bolus (1.0 μg/kg) plus infusion (0.5 μg/kg/h) | 42 ± 10 | 63.1 ± 11.6 | 164.2 ± 6.4 | 9/12 | I:15f II:5 |
Laparoscopic cholecystectomy 29 ± 11 |
Three‐step: IV ketorolac, IV tramadol, IV fentanyl at 30 minute intervals, if VAS pain > 4i | 2. Propofol 83 ± 23.4 mg, ketorolac 30 mg IV, dexamethasone 8 mg IV, 0.25% bupivacaine 3 mL SC and IC |
| Placebo (21) | Same rate and volume | 44 ± 14 | 68.9 ± 12.1 | 166.5 ± 7.3 | 10/11 | I: 14f II: 6 |
Laparoscopic cholecystectomy 29 ± 14 |
Same as above | Same as above, but propofol 117 ± 33.9 mg | |
|
Tufanogullari 2008 (80) USA |
DEX 0.2 (20) | Intravenous infusion (0.2 μg/kg/h) |
47 ± 10 | 127 ± 20 | 169 ± 10 | 3/17 | II: 6 III: 14 |
Laparoscopic gastric banding (12)/bypass (8) 110 ± 62 |
Three‐step: IV fentanyl, PCA morphine (2 mg bolus and 10 min lock‐out), oral hydromorphonej |
1. Midazolam 20 µg/kg IV. Celecoxib 400 mg orally 2. Less desflurane than placebo group, bupivacaine 0.25% at fascial level |
| DEX 0.4 (20) | Intravenous infusion (0.4 μg/kg/h) |
48 ± 9 | 138 ± 41 | 169 ± 8 | 4/16 | II: 2 III: 18 |
Laparoscopic gastric banding (9)/bypass (11) 107 ± 35 |
Same as above | Same as above | |
| DEX 0.8 (20) | Intravenous infusion (0.8 μg/kg/h) |
40 ± 10 | 151 ± 36 | 172 ± 13 | 9/11 | II: 4 III: 16 |
Laparoscopic gastric banding (9)/bypass (11) 111 ± 56 |
Same as above | Same as above | |
| Placebo (20) | Same rate and volume | 43 ± 16 | 127 ± 25 | 165 ± 12 | 3/17 | II: 6 III: 14 |
Laparoscopic gastric banding (9)/bypass (11) 116 ± 52 |
Same as above | Same as above, but more desflurane than in DEX groups | |
|
Xiao 2013 (120) China |
DEX plus remifentanil high‐dose (40) | Intravenous bolus DEX (1.0 μg/kg) plus infusion remifentanil (0.4 μg/kg/h) |
57 ± 11 | 57 ± 10 | NR | 17/23 | I‐II | Open abdominal surgery 204 ± 18 |
Morphine | 2. Less sevoflurane than in low‐dose remifentanil group |
| Placebo plus remifentanil high‐dose (40) | Intravenous bolus placebo plus infusion remifentanil (0.4 μg/kg/h) |
58 ± 12 | 58 ± 11 | NR | 18/22 | I‐II | Open abdominal surgery 198 ± 36 |
Morphine | 2. Less sevoflurane than in low‐dose remifentanil group | |
| Placebo plus remifentanil low‐doseb (40) | Intravenous bolus placebo plus infusion remifentanil (0.05 μg/kg/h) |
56 ± 13 | 59 ± 10 | NR | 18/22 | I‐II | Open abdominal surgery 198 ± 30 |
Morphine | 2. More sevoflurane than in other groups | |
Data are reported as mean ± SD (standard deviation). ASA and gender are reported as number (n) of participants
ASA: American Society of Anesthesiologists Physical Status 1 to 6, where 1 = Healthy person, 2 = Mild systemic disease, 3 = Severe systemic disease, 4 = Severe systemic disease that is a constant threat to life, 5 = A moribund person who is not expected to survive without the operation, 6 = A declared brain‐dead person whose organs are being removed for donor purposes; DEX: dexmedetomidine; NR: not reported; IV: intravenous; PCA: patient‐controlled analgesia; SC: subcutaneously; IC: intracutaneously
aProbably an error in height and weight; bData from this intervention arm were not included in our analysis; cError in report of gender; dASA not reported, but probably ASA I to II; ePlease note that many participants were judged to be ASA I despite having abdominal cancer; fError in reporting of ASA; gNot reported if laparoscopic or open; hCriteria for administering pentazocine described only as 'an extra dose of pentazocine on complaint of immediate postoperative pain'; iIn our data and analyses, we looked only at opioid, but amount of ketorolac IV was significantly different between groups with 43.5 ± 18 mg for DEX group and 66 ± 39.6 for placebo group (P value < 0.05). The amount of fentanyl was not reported, only that 2 participants from the placebo group needed it. Only amount of IV tramadol was collected by us, showing no significant difference between groups. jNo other description of criteria for administering 'rescue' analgesia. In postoperative care unit (PACU), pain was evaluated every 5 to 15 minutes, but after PACU discharge, no further description; kTotal dose of pentazocine was combination of intraoperative and postoperative amounts. Intraoperatively, the DEX group received pentazocine 0.3 mg/kg, whereas the placebo group received 0.5 mg/kg, and an additional bolus of 0.1 mg/kg was administered if signs of intraoperative pain
Participants
The review analysis included 422 participants. At least 254 were women (60.2%) and 148 men (35.1%); for the last 20 participants, we noted an error in reported data (see Table 3; Bakhamees 2007). Studies were conducted in Egypt (two studies, 140 participants; Bakhamees 2007; Mohamed 2012); USA (two studies, 100 participants; Feld 2006; Tufanogullari 2008); India (one study, 60 participants; Khanduja 2013); Korea (one study, 42 participants; Park 2012); and China (one study, 80 participants; Xiao 2013). Participants' mean age ranged from 29 to 58 years with an upper limit of 66 years of age and no exact report of lower limit (inclusion criteria from 18 years of age). Types of surgery included bariatric surgery (two studies with 100 participants having a gastric bypass and one study with 80 participants having gastric banding or gastric bypass; Bakhamees 2007; Feld 2006; Tufanogullari 2008); cholecystectomy (two studies, 102 participants; Khanduja 2013; Park 2012); and major abdominal cancer surgery (one study, 60 participants; Mohamed 2012). Four studies (Bakhamees 2007; Khanduja 2013; Park 2012; Tufanogullari 2008) included laparoscopic surgery (262 participants); two included open surgery (100 participants) (Feld 2006, Xiao 2013); and one study reported no details about type of surgery, other than that it was major abdominal cancer surgery (60 participants; Mohamed 2012). Studies included participants with American Society of Anesthesiologists Physical Status (ASA) I to III (the three studies with bariatric surgery included participants with ASA II to III, and the other studies included participants with ASA I to II). All studies including ASA II to III defined exclusion criteria comprising neurological, cardiovascular, respiratory, renal and hepatic disease. Other common exclusion criteria were alcohol and drug abuse and psychiatric disease.
See Characteristics of included studies and Table 3 for additional details.
Interventions
Six of the seven studies compared dexmedetomidine versus placebo or control (Bakhamees 2007; Khanduja 2013; Mohamed 2012; Park 2012; Tufanogullari 2008; Xiao 2013). One study (20 participants) compared dexmedetomidine with fentanyl (Feld 2006). In five of seven studies (Bakhamees 2007; Feld 2006; Khanduja 2013; Park 2012; Tufanogullari 2008), dexmedetomidine was administered intravenously as an infusion intraoperatively (three studies initiated the infusion by a bolus; Bakhamees 2007; Feld 2006; Park 2012), in another study, solely as an intravenous bolus before induction of anaesthesia (Xiao 2013) and in another study (60 participants), as a bolus intrathecally before induction of anaesthesia (Mohamed 2012). Dosage for the intravenous bolus ranged from 0.5 μg/kg to 1.0 μg/kg. The intrathecally administered bolus was a dosage of 5 μg. Dosage of intravenous infusion ranged from 0.4 to 0.6 μg/kg/h, except for the study which included three dose regimens of 0.2, 0.4 and 0.8 μg/kg/h (Tufanogullari 2008), which were pooled to a single intervention group in our analysis.
See Characteristics of included studies and Table 3 for details. The table describes the actual interventions provided in the study, regardless of our decisions to exclude or pool intervention groups.
Outcomes
See Characteristics of included studies and Table 3 for additional details. The table describes only the outcomes relevant for our review. All studies reported other outcomes, and some studies had several primary outcomes not reported in this review. In the following paragraphs, we will present a resume of the characteristics of our primary and secondary outcomes.
Primary outcomes
Six out of seven studies contributed to our primary outcomes (Bakhamees 2007; Feld 2006; Mohamed 2012; Park 2012; Tufanogullari 2008; Xiao 2013). Time points selected for our primary outcomes showed variability as reported by included studies. We decided post hoc, but before looking further into the actual results, that variation of one, two, three and six hours was acceptable for our prespecified time points, respectively, three, six, 12 and 24 hours postoperatively. One study reported 'postoperative day one' instead of 24 hours (Tufanogullari 2008), and two studies reported the amount of 'rescue' opioid and VAS pain at two hours after surgery instead of at three hours (Bakhamees 2007; Feld 2006). We have reported these data without adjustments.
Concerning the opioid‐sparing effect of dexmedetomidine or the amount of 'rescue' opioid, four studies reported monotherapy analgesic regimens consisting of PCA (patient‐controlled analgesia) morphine (Bakhamees 2007; Feld 2006), intravenous morphine with no further specification (Xiao 2013) and intravenous tramadol (Mohamed 2012). Two studies had three‐step 'rescue' analgesia regimens; one consisted of intravenous fentanyl, intravenous PCA morphine and oral hydromorphone‐acetaminophen (Tufanogullari 2008), and the other consisted of oral ketorolac, intravenous tramadol and intravenous fentanyl (Park 2012). We converted all types of opioid to the equianalgesic intravenous dose of morphine on the basis of the following equivalents, when approximately:
1 mg intravenous morphine equals
21 mg intravenous tramadol (30 mg oral tramadol) equals
0.02 mg (20 μg) intravenous fentanyl equals
0.75 mg oral hydromorphone
(emedicine.medscape.com; globalrph.com; healthquality.va.gov; irf.dk; en.wikipedia.org/wiki/equianalgesic; tramadolfacts.com).
If a study used paracetamol or a non‐steroidal anti‐inflammatory drug (NSAID), we did not include these data in our analysis but presented them only in a narrative report. One study reported only the total dose of intraoperative and postoperative pentazocine; we reported this information narratively as well, along with the outcome of proportion of participants needing 'rescue' analgesia (Khanduja 2013).
Concerning the analgesic efficacy of dexmedetomidine measured on a visual analogue scale (VAS) for pain, one study used a verbal rating scale (VRS 0 to 10) (Tufanogullari 2008), and the other used a VAS. Two studies used median and interquartile range (IQR) (Bakhamees 2007; Feld 2006), which we converted to mean and standard deviation (SD), making the assumption that the distribution of VAS is symmetrical and similar to a normal distribution. The median was thus directly used as a mean, and the IQR was assumed to be equal to an SD of 1.35 (chapter 7.7.5.3. Higgins 2011). One study reported VAS pain at two and four hours after surgery instead of at three hours, and we chose to report the mean of these two time points.
Secondary outcomes
Five studies contributed to our secondary outcomes, of which one reported time to first request of 'rescue' analgesia (Mohamed 2012); two reported proportion of participants needing 'rescue' analgesia (Khanduja 2013; Tufanogullari 2008); one reported postoperative sedation 12 hours after surgery measured by the Ramsay Sedation Scale (Xiao 2013); three reported PONV (Bakhamees 2007; Mohamed 2012; Tufanogullari 2008); one reported time to first passage of flatus (Tufanogullari 2008) and one reported time to first out‐of‐bed mobilization (reported as time to ambulation by study authors; Tufanogullari 2008). Mohamed 2012 reported postoperative sedation with no time point specified and nothing else other than 'no significance between groups'. No studies reported time to first passage of stool.
Concerning the proportion of participants with PONV, all three studies with this outcome divided PONV into participants with nausea and participants with vomiting (Bakhamees 2007; Mohamed 2012; Tufanogullari 2008). We chose to report only on participants with nausea and excluded those with vomiting to avoid making a unit of analysis error. One study (Tufanogullari 2008) reported number of participants needing 'rescue' antiemetic therapy, as well as nausea scores (verbal rating scale 0 to 10), during the first hour postoperatively. We presented this additional information narratively.
Excluded studies
We excluded 32 studies for specific reasons mentioned under Characteristics of excluded studies. This table comprises the studies that might appear to meet eligibility criteria but were excluded after a closer look at the full‐text article. (For details of the reasons for exclusion by group, see Figure 1.)
We excluded studies without a control group. Akinci 2011 exemplified this, including three intervention groups with dexmedetomidine but no control group.
We excluded studies in which dexmedetomidine was not the sole difference between intervention groups, or in which dexmedetomidine was not compared with another single drug, to avoid any synergy between dexmedetomidine and another drug. Málek 2010 and Marangoni 2005 exemplified this.
We excluded studies with procedures such as hernia repair, gynaecological surgery, urological surgery, procedures to the kidneys and vascular surgery. We planned to include surgery to the spleen in our review; however no studies examined this type of procedure (except one study awaiting classification; Unlugenc 2005). Gupta 2014b provided mixed surgery, including plastic and otorhinolaryngological surgery, but also regional 'general surgery' of about one hour duration. We contacted the study authors by email to confirm which type, but we received no reply. We excluded this study because it probably did not examine abdominal surgery.
We excluded studies with outcomes relevant to our review if no reports described outcomes at the specific time points prespecified in our protocol (Jessen 2013) ‐ with particular variance (one, two, three and six hours of variance for three, six, 12 and 24 hours, respectively). Harsoor 2014 exemplified this.
Studies awaiting classification
Thirteen studies are awaiting classification because we needed additional information from study authors,and we have had no success when attempting to correspond. (See Characteristics of studies awaiting classification for further details.)
We would have included Ceballos 2011 in our review, but because VAS postoperative pain was reported as a dichotomous value ('no pain' = VAS 0 to 4, 'pain' = VAS 5 to 10) and actually measured values were not included, we categorized this study as awaiting classification. Study authors did not report type or amount of 'rescue' analgesia. We contacted these authors to request the appropriate data, and if and when they reply, we will include this study in the next updated version of this review. This study also reported postoperative sedation, but not at or near a time point prespecified by our review.
Yacout 2012 reported VAS pain as a mean with no variance at all (no standard deviation, P value or other). We contacted study authors without success. We could have imputed a variance, but we chose to let the study be described as unclassified, because a statistical analysis of VAS pain for this study would be problematic at any rate as the 'rescue' analgesia used was not an opioid but was ketorolac. An NSAID is not directly convertible to opioid; therefore this study did not contribute to a statistical analysis for our other main outcome, the opioid‐sparing effect of dexmedetomidine, and our two main outcomes cannot be interpreted to our satisfaction one without the other.
Unlugenc 2005 included mixed surgery, including mini‐laparotomy, cholecystectomy, splenectomy and inguinal, incisional or umbilical hernia repair. We contacted study authors to request individual participant data for the three groups that did not undergo hernia repair, but without success. The same problem applied to five other studies (Arain 2004; Bicer 2006; Kilicaslan 2006; Mizrak 2010; Scheinin 1992) for which we also contacted first authors without success. One other study looked at lower abdominal surgery without further definition (both men and women included), and we contacted the first study author for details, without success (Altindis 2008).
Four studies could not be classified because we could not retrieve the full text, and our attempts to contact study authors were unsuccessful (Anvaroglu 2008; Kordan 2006; Subasi 2012; Yektas 2011).
Ongoing studies
We identified six ongoing studies. See Characteristics of ongoing studies for details.
Awad 2014 (recruiting participants) compares three different doses of dexmedetomidine with the primary outcomes of shivering and quality of emergence from anaesthesia. This study might not be relevant for this review as no outcomes and time points seem to correspond directly to ours. The same applies to Jung 2014, which compared anaesthesia with (1) sevoflurane, (2) propofol and remifentanil, (3) sevoflurane and dexmedetomidine and (4) propofol, remifentanil and dexmedetomidine. Jung et al did not specify outcomes relevant for this review, other than 'safety', which we presume translates to complications or adverse effects. The type of surgery is not specified as other than abdominal surgery. Kim 2014 is investigating the effect of dexmedetomidine in combination with fentanyl on pain after surgery for colon cancer. This study is registered as completed and has not been verified since March 2012. We await results from this study. Wai 2014 is investigating the effect of morphine and COX‐2 inhibitor with or without dexmedetomidine on pain after colorectal cancer surgery. These trial authors did not specify time points for assessment of postoperative pain, but given that follow‐up is five days, they probably will report values for one or more time points relevant to this review. They also report on flatus, which is a relevant outcome for our review that has been assessed by few studies. Yoo 2014 is investigating the effect of dexmedetomidine on gastrointestinal function after laparoscopic gastrectomy due to gastric cancer. They have been recruiting participants since June 2014. Zeeni 2014 is also investigating dexmedetomidine for analgesia after bariatric surgery (laparoscopic sleeve gastrectomy). This study has several outcomes relevant to this Cochrane review, and it is currently recruiting participants (starting in August 2014); we await study results.
Risk of bias in included studies
For a detailed argumentation for each study's risk of bias, please see the risk of bias tables under Characteristics of included studies. For an overview of the risk of bias for all domains and outcomes and for all studies, please see Figure 2 and Figure 3.
2.
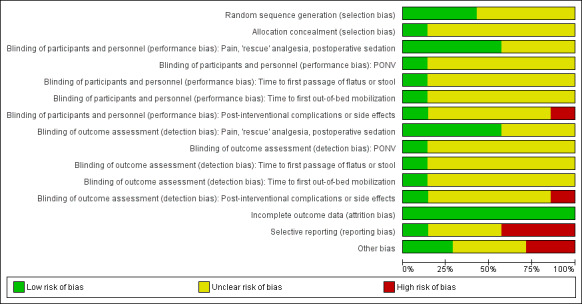
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
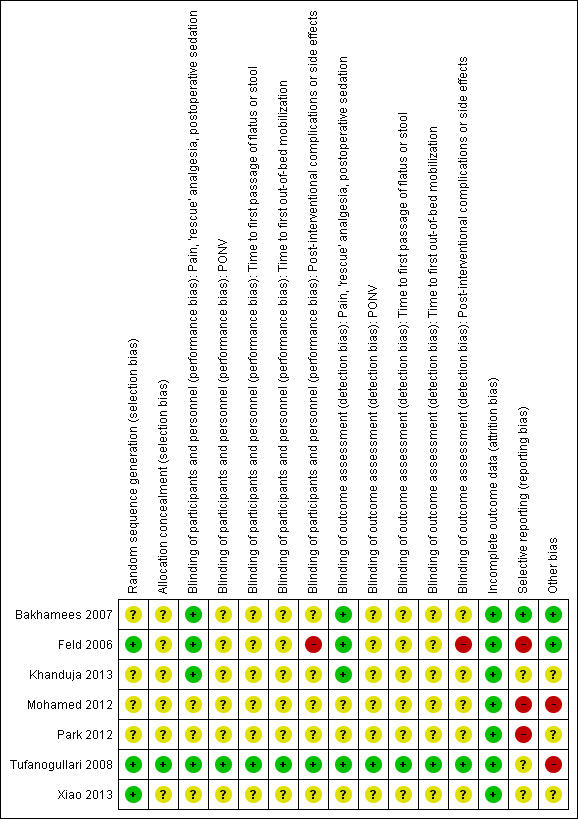
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Please note that the yellow colour in the figures, signifying 'unclear risk of bias', does not permit a distinction between 'not relevant' and 'not sufficient information'.
Allocation
Assessment of risk of selection bias consists of a judgement of how the random sequence was generated and how the allocation was concealed from participants and from investigators enrolling participants.
Three out of seven studies used a computer‐generated random sequence or random number table and were judged to have low risk of bias (Feld 2006; Tufanogullari 2008; Xiao 2013), whereas the remaining four studies presented insufficient information to enable us to make a judgement. Concerning allocation concealment, only one study provided enough information to allow us to make a judgement, which indicated low risk of bias (Tufanogullari 2008).
See Characteristics of included studies for additional details.
Blinding
One study was convincingly double‐blinded and described details of how this was done (Tufanogullari 2008); therefore we assigned this study as having low risk of bias for all outcomes.
Two studies included a non‐blinded anaesthesiologist but blinded participants with blinding of some of the personnel assessing outcomes (Bakhamees 2007; Feld 2006). A non‐blinded anaesthesiologist might lead to a difference in the administration of anaesthetics, but for one study, such a difference would lead to an underestimation of effect (Bakhamees 2007), and for the other study, it probably would not influence effects on pain and 'rescue' analgesia postoperatively (Feld 2006). Therefore, both studies had low risk of bias concerning pain and 'rescue' analgesia. When it comes to post‐interventional complications and side effects, a non‐blinded anaesthesiologist caused high risk of bias for the complications reported in one study (Feld 2006), whereas the other study reported no complications (Bakhamees 2007). See Characteristics of included studies for additional details.
One study was single‐blinded and reported no further details, probably including blinded participants and a non‐blinded anaesthesiologist. As the time of follow‐up was about 30 minutes postoperatively, it was probably the anaesthesiologist who assessed all postoperative outcomes. The non‐blinded anaesthesiologist might have had an influence on the measured difference in amount of analgesia given intraoperatively, but as this would lead to an underestimation of effect, risk of bias was low concerning postoperative pain and 'rescue' analgesia (Khanduja 2013). See Characteristics of included studies for additional details. Three studies did not provide enough information about blinding to enable us to make a clear judgement (Mohamed 2012; Park 2012; Xiao 2013).
Incomplete outcome data
All studies had low risk of bias concerning incomplete outcome data. Only Tufanogullari 2008 had excluded participants from the final analysis ‐ one in each of the three dose‐differentiated dexmedetomidine intervention groups. Exclusion was result of surgical reasons in all three cases; therefore, we have judged risk of bias as low.
See Characteristics of included studies for additional details.
Selective reporting
For one of the seven included trials, we found a published protocol (Tufanogullari 2008). However, this study had unclear risk of selective reporting because outcomes prespecified in the protocol were described in very general terms only. Two outcomes seemed to be added post hoc (number of participants discharged on postoperative day one and number of days until discharge), but they were secondary outcomes and showed no significant differences between groups; therefore, risk of bias was not judged as high.
Only one study had low risk of selective reporting bias (Bakhamees 2007). Even though the protocol was not available, outcomes described in the study's Methods section and outcomes reported in the study's Results section corresponded.
The remaining studies had unclear (Khanduja 2013; Xiao 2013) or high risk of selective reporting bias (Feld 2006; Mohamed 2012; Park 2012) because information was insufficient to enable us to make a judgement, or because substantial irregularities were apparent between what was prespecified in the Methods section and what was actually reported in the Results section.
See Characteristics of included studies for additional details.
Other potential sources of bias
Two studies were judged as having high risk of other bias. Mohamed 2012 had remarkably low standard deviations for the amount of 'rescue' opioid (see Analysis 1.6), making detailed information important about differences in anaesthetic agents between groups and other analgesic drugs used. Furthermore, the study provided no details about which type of surgery was performed, other than that it was major abdominal cancer surgery. We attempted, without success, to contact the first author of this study.
1.6. Analysis.
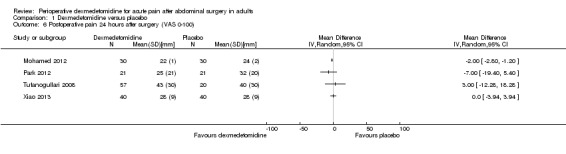
Comparison 1 Dexmedetomidine versus placebo, Outcome 6 Postoperative pain 24 hours after surgery (VAS 0‐100).
We judged Tufanogullari 2008 as having high risk of other bias because criteria for the use of the three‐step 'rescue' analgesia regimen were not clearly described. Furthermore, investigators did not describe the information given to participants on how to administer the analgesia at home. Therefore, one might suspect that the study lacked control of participant compliance at home, leading to potential contamination of outcomes. This study was sponsored in part by a medical company that manufactured dexmedetomidine, but it was unclear if this relationship introduced other potential bias. We contacted the first study author to request further information, without success.
For three studies, information was insufficient to enable us to make a clear judgement (Khanduja 2013; Park 2012; Xiao 2013). Two studies seemed to have no other apparent bias (Bakhamees 2007; Feld 2006). Only Tufanogullari 2008 reported a potential conflict of interest.
See Characteristics of included studies for additional details.
Effects of interventions
See: Table 1
Six (Bakhamees 2007; Khanduja 2013; Mohamed 2012; Park 2012; Tufanogullari 2008; Xiao 2013) of the seven included studies compared dexmedetomidine with placebo, whereas one study compared dexmedetomidine with fentanyl (Feld 2006).
See Table 1, Data and analyses and Table 4 for additional details.
3. Results of included studies.
| Comparison: dexmedetomidine versus placebo | ||||||
| Bakhamees 2007 | Mohamed 2012 | Park 2012 | Tufanogullari 2008 | Khanduja 2013 | Xiao 2013 | |
| Number of participants included in this review | 80 | 60 | 42 | 77 (80)i | 60 | 80 |
| Outcome | ||||||
| 'Rescue' morphine (intravenous, mg) 3 hours after surgery (MD, 95% CI) | ‐5.20 (‐5.79, ‐4.61)*a | NR | NR | ‐3.65 (‐6.04, ‐1.26)*b | NR | NR |
| 'Rescue' morphine (intravenous, mg) 6 hours after surgery (MD, 95% CI) | NR | NR | NR | NR | NR | NR |
| 'Rescue' morphine (intravenous, mg) 12 hours after surgery (MD, 95% CI) | NR | NR | NR | NR | NR | NR |
| 'Rescue' morphine (intravenous, mg) 24 hours after surgery (MD, 95% CI) | ‐12.00 (‐15.10, ‐8.90)* | ‐7.20 (‐7.48, ‐6.92)* | 0.10 (‐0.95, 1.15)c | ‐56.00 (‐112.01, 0.01)*f | NR | NRj |
| VAS pain 0‐100 (mm) 3 hours after surgery (MD, 95% CI) | ‐30.00 (‐38.25, ‐21.75)*d | ‐2.00 (‐3.29, ‐0.71)*e | NR | NR | NR | NR |
| VAS pain 0‐100 (mm) 6 hours after surgery (MD, 95% CI) | NR | ‐3.00 (‐5.95, ‐0.05)* | ‐2.00 (‐11.02, 7.02) | NR | NR | NR |
| VAS pain 0‐100 (mm) 12 hours after surgery (MD, 95% CI) | NR | ‐6.00 (‐6.72, ‐5.28)* | NR | NR | NR | ‐2.00 (‐6.61, 2.61)j |
| VAS pain 0‐100 (mm) 24 hours after surgery (MD, 95% CI) | NR | ‐2.00 (2.80, ‐1.20)* | ‐7.00 (‐19.40, 5.40) | 3.00 (‐12.28, 18.28)f | NR | 0.00 (‐3.94, 3.94)j |
| Time to first request of 'rescue' analgesia (hours, MD, 95% CI) | NR | 3.07 (2.76, 3.38)* | NR | NR | NR | NR |
| Proportion of participants needing 'rescue' analgesia (RR, 95% CI) | NR | NR | NR | 1.00 (0.93, 1.07) | 0.44 (0.15, 1.29) | NR |
| Postoperative sedation 12 hours after surgery (RSS, Ramsay Sedation Scale from 1 = Anxious to 6 = Unresponsive) (MD, 95% CI) | NR | NR | NR | NR | NR | 1.60 (1.49, 1.71)† |
| Porportion of participants with PONV (RR, 95% CI) | 0.67 (0.12, 3.78)g | 0.50 (0.17, 1.48)g | NR | 0.54 (0.33, 0.87)*g | NR | NR |
| Time to first passage of flatus/stool (hours, MD, 95% CI) | NR | NR | NR | 5.00 (‐5.60, 15.60) h | NR | NR |
| Time to first out‐of‐bed mobilization (hours, MD, 95% CI) | NR | NR | NR | ‐0.33 (‐3.95, 3.29) | NR | NR |
| Comparison: dexmedetomidine versus fentanyl | ||||||
| Feld 2006 | ||||||
| Number of participants | 20 | |||||
| Outcome | ||||||
| 'Rescue' morphine (mg) 3 hours after surgery (MD, 95% CI) | ‐8.50 (‐12.75, ‐4.25)* | |||||
| VAS pain 0‐100 (mm) 3 hours after surgery (MD, 95% CI) | ‐40.00 (‐51.53, ‐28.47)*d | |||||
* Significant difference between groups in favour of dexmedetomidine (P value < 0.05)
† Significant difference between groups in favour of control (P value < 0.05)
MD: mean difference; CI: confidence interval; VAS: visual analogue scale; RR: risk ratio; PONV: postoperative nausea and vomiting; NR: no data reported; aTime point at 2 hours after surgery; bNo specific postoperative time point reported, but only 'rescue' opioid (fentanyl) during initial time in PACU, given by personnel until PCA morphine possible; cThese data reflected only the amount of intravenous tramadol given to participants, if intravenous ketorolac was not efficient. The dexmedetomidine group had smaller consumption of ketorolac (mg) with a mean difference of ‐22.50 (95% CI ‐41.10 to ‐3.90). If intravenous tramadol still was not sufficient to relieve pain, fentanyl was administered. This was necessary for 2 participants only, in the placebo group, but the dose was not reported; dTime point 2 hours after surgery, and data converted from median and interquartile range (IQR) by presuming mean and median to be equal, and standard deviation times 1.35 to be equal to IQR; eTime points reported were 2 and 4 hours after surgery, and we have reported the mean; fTime point was 'postoperative day one', which could extend some hours beyond 24 hours after surgery; gNausea and vomiting were reported separately. We reported only nausea, presuming that participants with vomiting also had nausea. Tufanogullari 2008 also found reduced need for 'rescue' antiemetics at the first postoperative day (RR 0.35, 95% CI 0.20 to 0.60) together with slightly reduced intensity of nausea on a verbal rating scale (VRS) 0‐10 compared with placebo during the first 30 minutes postoperatively; hOnly report of flatus, not stool; iPostoperative data from 3 participants were excluded from the final analysis, 1 from each of the 3 intervention groups (pooled to 1 intervention group in our review), because of surgical complications discovered at the postsurgical ward. Number of participants was therefore 77 for all outcomes, except for post‐interventional complications/adverse effects, for which it was 80 (not reported in this table, but in review text); jThis trial reported amount of 'rescue' morphine at 48 hours after surgery only, showing a significant difference (mg, mean, SD) between the dexmedetomidine plus high‐dose remifentanil group (54 ± 13) versus the placebo plus high‐dose remifentanil group (78 ± 24), with mean difference of ‐21.00 (95% CI ‐29.46 to ‐12.54). Reported VAS pain scores should be interpreted with this information
Comparison 1. Dexmedetomidine versus placebo
For all outcomes in this comparison, we considered performing meta‐analyses unsuitable. For our main outcomes at 24 hours after surgery, the quantity of data seemed sufficient to be pooled. However, for the amount of 'rescue' opioid, heterogeneity was considered substantial clinically and statistically (I2 statistic = 98%); therefore a meta‐analysis was inappropriate (for additional details about clinical and methodological heterogeneity between studies, see Quality of the evidence). For the outcome of postoperative pain 24 hours after surgery, four studies (Mohamed 2012; Park 2012; Tufanogullari 2008; Xiao 2013) reported data, and statistical heterogeneity was not substantial (I2 statistic = 0%). Nevertheless, we considered a meta‐analysis to be inappropriate for this outcome because we judged clinical heterogeneity to be substantial. We made this judgement mainly for two reasons.
When heterogeneity was considered substantial for the outcome of amount of 'rescue' analgesia, it seemed reasonable to regard VAS postoperative pain as equally heterogeneous (the studies were almost the same at 24 hours after surgery).
The standard deviation reported for the study with highest statistical weight seemed clinically unrealistic (Mohamed 2012).
For the proportion of participants with PONV, we considered a meta‐analysis inappropriate because a relatively small quantity of data was combined with imprecision in collection of results. For all other outcomes, the quantity of data was too sparse to permit meta‐analyses.
Five studies (Bakhamees 2007; Mohamed 2012; Park 2012; Tufanogullari 2008; Xiao 2013) reported data for our two primary outcomes, although not for all time points, and five studies (Bakhamees 2007; Khanduja 2013; Mohamed 2012; Tufanogullari 2008; Xiao 2013) contributed to some of our secondary outcomes. One study (Khanduja 2013) reported only one outcome collected by us: the proportion of participants needing 'rescue' analgesia ‐ a secondary outcome that we had not prespecified to be included in the Table 1.
Primary outcomes
The opioid‐sparing effect of dexmedetomidine (amount of 'rescue' opioid)
At three hours after surgery, we found low‐quality evidence that intravenous morphine equivalent consumption was reduced with dexmedetomidine, with a mean difference of ‐5.20 mg (95% confidence interval (CI) ‐5.79 to ‐4.61) for Bakhamees 2007 (80 participants) and ‐3.65 mg (95% CI ‐6.04 to ‐1.26) for Tufanogullari 2008 (77 participants) ‐ respectively, 51% and 39% reduction. I2 statistic = 34%. See Analysis 1.1. We downgraded the quality of evidence by two levels because of some study limitations and imprecision (small quantity of data).
1.1. Analysis.
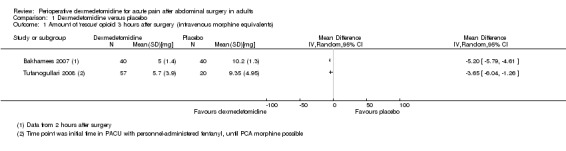
Comparison 1 Dexmedetomidine versus placebo, Outcome 1 Amount of 'rescue' opioid 3 hours after surgery (intravenous morphine equivalents).
No studies reported data for the time points six and 12 hours after surgery.
At the time point 24 hours after surgery, we found very low‐quality evidence that intravenous morphine equivalent consumption was reduced with dexmedetomidine, with a mean difference of ‐12.00 mg (95% CI ‐15.10 to ‐8.90) ‐ a 25% reduction ‐ for Bakhamees 2007 (80 participants); ‐7.20 mg (95% CI ‐7.48 to ‐6.92) ‐ a 54% reduction ‐ for Mohamed 2012 (60 participants); and ‐56.00 mg (95% CI ‐112.01 to 0.01) ‐ a 26% reduction ‐ for Tufanogullari 2008 (77 participants). One study, Park 2012 (42 participants), was more difficult to quantify; investigators provided a three‐step analgesia regimen, with a significant difference in the need for ketorolac (first step) favouring dexmedetomidine (intravenous ketorolac, mg, mean difference (MD) ‐22.50, 95% CI ‐41.10 to ‐3.90), no significant difference in the need for tramadol (second step) (converted to intravenous morphine, mg, MD 0.10, 95%CI ‐0.95 to 1.15) and no significant difference in the number of participants requiring fentanyl (third step) (risk ratio (RR) 0.18, 95% CI 0.01 to 4.02) (see Analysis 1.2). We downgraded the quality of evidence by three levels, from high to very low, because of serious study limitations, heterogeneity (I2 statistic = 98%) and imprecision of results (broad statistical variance).
1.2. Analysis.
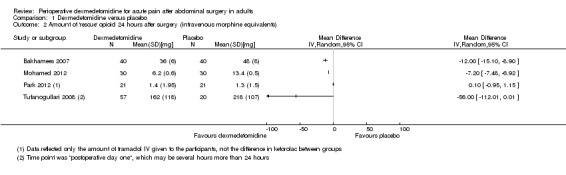
Comparison 1 Dexmedetomidine versus placebo, Outcome 2 Amount of 'rescue' opioid 24 hours after surgery (intravenous morphine equivalents).
Estimated effects should be evaluated together with VAS postoperative pain; all of the above‐mentioned studies reported reduced intensity of pain with dexmedetomidine or no significant difference compared with placebo (at varying time points). See the section below.
The analgesic efficacy of dexmedetomidine (VAS postoperative pain)
At three hours after surgery, we found very low‐quality evidence for a reduction in VAS postoperative pain (0 to 100 mm) with dexmedetomidine, with a mean difference of ‐30.00 mm (95% CI ‐38.25 to ‐21.75) for Bakhamees 2007 (80 participants), and a clinically unimportant mean difference of ‐2.00 mm (95% CI ‐3.29 to ‐0.71) for Mohamed 2012 (60 participants). See Analysis 1.3. We downgraded the quality of evidence by three levels because of serious study limitations, imprecision (small amount of data) and heterogeneity of results (I2 statistic = 98%).
1.3. Analysis.
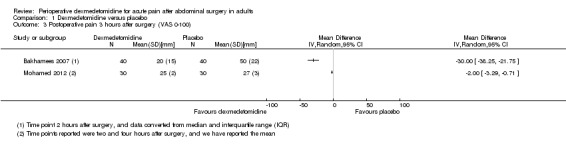
Comparison 1 Dexmedetomidine versus placebo, Outcome 3 Postoperative pain 3 hours after surgery (VAS 0‐100).
At six hours after surgery, we found very low‐quality evidence for clinically unimportant mean differences in VAS postoperative pain for two studies (a slight reduction of ‐3.00 mm (95% CI ‐5.95 to ‐0.05) with dexmedetomidine for Mohamed 2012 (60 participants), and a mean difference of ‐2.00 mm (95% CI ‐11.02 to 7.02) for Park 2012 (42 participants) favouring neither group). I2 statistic = 0%. See Analysis 1.4. We assessed the quality of evidence as very low because of serious study limitations and imprecision (small quantity of data).
1.4. Analysis.
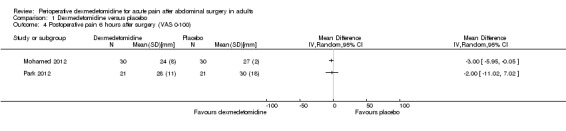
Comparison 1 Dexmedetomidine versus placebo, Outcome 4 Postoperative pain 6 hours after surgery (VAS 0‐100).
At 12 hours after surgery, results were much the same as for VAS postoperative pain at six hours after surgery, with very low‐quality evidence for mean differences of less clinical importance from two studies (a modest reduction of ‐6.00 mm (95% CI ‐6.72 to ‐5.28) with dexmedetomidine for Mohamed 2012 (60 participants), and a mean difference of ‐2.00 mm (95% CI ‐6.61 to 2.61) for Xiao 2013 (80 participants) favouring neither group). I2 statistic = 65%. See Analysis 1.5. We downgraded the quality of evidence for the same reasons.
1.5. Analysis.
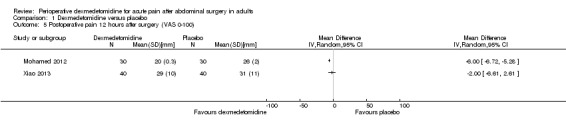
Comparison 1 Dexmedetomidine versus placebo, Outcome 5 Postoperative pain 12 hours after surgery (VAS 0‐100).
At 24 hours after surgery, the quantity of data was larger than for the other time points, but the quality was equally very low for evidence of mean differences in VAS pain close to favouring neither dexmedetomidine nor placebo (‐7.00 mm (95% CI ‐19.40 to 5.40) for Park 2012 (42 participants), 3.00 (95% CI ‐12.28 to 18.28) for Tufanogullari 2008 (77 participants), 0.00 (95% CI ‐3.94 to 3.94) for Xiao 2013 (80 participants) and ‐2.00 (95% CI ‐2.80 to ‐1.20) for Mohamed 2012 (60 participants)). I2 statistic = 0%. See Analysis 1.6. The quality of evidence was downgraded by three levels because of serious study limitations, but also for the same reasons (heterogeneity and imprecision) that led to downgrading of quality of the evidence for the amount of 'rescue' analgesia at 24 hours after surgery (given that the two outcomes are closely interrelated and that studies reporting data at 24 hours after surgery were almost the same).
As mentioned, postoperative pain effect estimates should be evaluated together with the amount of 'rescue' analgesia, and all studies reporting VAS postoperative pain also reported amount of 'rescue' analgesia to be reduced with dexmedetomidine compared with placebo (see the section above). However, the amount of 'rescue' analgesia for Xiao 2013 was reported only at 48 hours after surgery and therefore was not reported by us, but study authors' report of VAS postoperative pain at 12 and 24 hours should be interpreted together with the information that the study found a reduction in amount of 'rescue' morphine at 48 hours after surgery in favour of dexmedetomidine, with a mean difference of ‐21.00 mg (95% CI ‐29.46 to ‐12.54).
Secondary outcomes
Regarding time to first request of ‘rescue’ analgesia, we assessed the quality of evidence as very low for the increase in time found for dexmedetomidine, with a mean difference of 3.07 hours (95% CI 2.76 to 3.38) (Mohamed 2012, 60 participants). The quality of evidence was downgraded because of serious study limitations and imprecision of results (small quantity of data).
For the proportion of participants needing ‘rescue’ analgesia, we found very low quality of evidence for effect estimates of this outcome. In one study (Khanduja 2013, 60 participants), follow‐up was very short after end of surgery (probably around 30 minutes); we did not consider this sufficient to conclude how many participants needed 'rescue' analgesia. However, had the study evaluated the time to need for 'rescue' analgesia, one might imagine that a significant effect could be found in favour of dexmedetomidine, particularly because the dexmedetomidine group received a significantly larger amount of intraoperative analgesia than the placebo group, thus underestimating the reported effect estimate (RR 0.44, 95% CI 0.15 to 1.29) in favour of dexmedetomidine for the outcome proportion of participants needing 'rescue' analgesia. The other study reporting data (Tufanogullari 2008, 77 participants) found that all participants without exception needed 'rescue' analgesia (with seven days of follow‐up); therefore we did not consider this study to be suitable for an evaluation of this outcome either, because apparently, the type of surgery and the type of anaesthesia in all circumstances would create a need for postoperative 'rescue' analgesia.
Regarding postoperative sedation, data were reported only at 12 hours after surgery, and we found very low‐quality evidence of an increased level of sedation with dexmedetomidine, with a mean difference in Ramsay Sedation Scale (RSS) 1 to 6 of 1.60 units (95% CI 1.49 to 1.71) (Xiao 2013, 80 participants). Reasons for downgrading one level were study limitations and imprecision of results (small quantity of data).
For the number of participants with PONV, we found low‐quality evidence of reduced risk with dexmedetomidine in one study (RR 0.54, 95% CI 0.33 to 0.87) for Tufanogullari 2008 (77 participants) and risk ratios in two studies close to favouring neither dexmedetomidine nor placebo (RR 0.67, 95% CI 0.12 to 3.78 for Bakhamees 2007 (80 participants); RR 0.50, 95% CI 0.17 to 1.48 for Mohamed 2012 (60 participants)). I2 statistic = 0%. See Analysis 1.7. The quality of evidence was downgraded by two levels because of study limitations and imprecision in reporting of results,as all three studies reported nausea and vomiting as separate outcomes, with the risk that some participants counted double with pooling of outcomes to PONV (unit of analysis error), or that cases were underreported if only nausea was reported as PONV. The study that found reduced risk of PONV with dexmedetomidine also found reduced need for 'rescue' antiemetics at the first postoperative day (RR 0.35, 95% CI 0.20 to 0.60) together with slightly reduced intensity of nausea on a verbal rating scale (VRS) of 0 to 10 compared with placebo during the first 30 minutes postoperatively (Tufanogullari 2008, 77 participants).
1.7. Analysis.
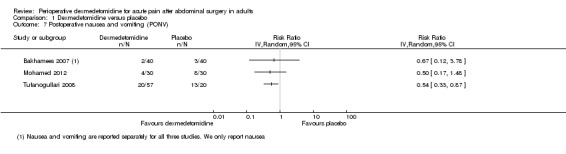
Comparison 1 Dexmedetomidine versus placebo, Outcome 7 Postoperative nausea and vomiting (PONV).
For time to first passage of flatus (no data for stool), low‐quality evidence suggested a mean difference close to favouring neither dexmedetomidine nor placebo, with reduction in time for the placebo group of 5.00 hours (95% CI ‐5.60 to 15.60) compared with dexmedetomidine, found in the only study reporting data (Tufanogullari 2008, 77 participants). Downgrading of two levels was imposed because of imprecision (small quantity of data).
Regarding time to first out‐of‐bed mobilization, low‐quality evidence suggested a mean difference in favour of neither dexmedetomidine nor placebo, with a mean difference of ‐0.33 hours after surgery (95% CI ‐3.95 to 3.29) for Tufanogullari 2008 (77 participants). Quality of evidence was again downgraded because of imprecision (small quantity of data).
Concerning post‐interventional complications or adverse effects, Tufanogullari 2008 (80 participants) reported the number of participants with hypotension requiring intervention (phenylephrine) other than a decrease in anaesthetic agents and a 200 mL fluid bolus, including two participants (10%) in the dexmedetomidine (DEX) 0.2 group, four (20%) in the DEX 0.4 group and 10 (50%) in the DEX 0.8 group, with the latter showing a risk ratio of 2.50 (95% CI 0.94 to 6.66) compared with the control group with four out of 20 (20%) participants. Tufanogullari 2008 also reported that the number of participants with hypertension and/or tachycardia requiring intervention (labetalol) other than an increase in anaesthetic agents (desflurane) was three (15%) in the DEX 0.2 group, one (5%) in the DEX 0.4 group and zero out of 20 (0%) in the DEX 0.8 group, with the latter showing a risk ratio of 0.09 (95% CI 0.01 to 1.54) compared with the control group with five out of 20 participants (25%). No other studies within this comparison reported side effects not already accounted for. The quality of evidence for these side effects was considered low, with downgrading by two levels due to imprecision of results. A slight tendency toward a probable dose‐response gradient may be present, but because of imprecision of results we have not upgraded the quality of evidence. See Characteristics of included studies.
Comparison 2. Dexmedetomidine versus fentanyl
Only one study reported data for this comparison (Feld 2006, 20 participants). Because of this small quantity of data, we did not present this comparison in our 'Summary of findings' table.
For our primary outcomes, the study reported data for the time point two hours after surgery, which we have accepted as a tolerable variation from our time point of three hours after surgery. For the amount of 'rescue' analgesia, consumption of intravenous morphine was reduced with dexmedetomidine, with a mean difference of ‐8.50 mg (95% CI ‐12.75 to ‐4.25). For VAS postoperative pain, intensity was reduced with dexmedetomidine, with a mean difference of ‐40.00 mm (95% CI ‐51.53 to ‐28.47). The quality of evidence for these estimates was considered low and was downgraded by two levels because of imprecision (small quantity of data).
For our secondary outcomes, investigators provided data only for post‐interventional complications/side effects. This study reported the number of participants with hypotension requiring intervention (epinephrine) as three out of 10 in the dexmedetomidine group versus one out of 10 in the fentanyl group (RR 3.00, 95% CI 0.37 to 24.17). Another complication reported was prolonged mechanical ventilation in one out of 10 participants in the fentanyl group versus null in the dexmedetomidine group (RR 0.33, 95% CI 0.02 to 7.32). We considered the quality of evidence for these outcomes as very low, downgraded by three levels because of high risk of bias pertaining to an unblinded anaesthesiologist (see Characteristics of included studies) and because of imprecision (small quantity of data).
Discussion
Summary of main results
See Table 1, Table 3 and Table 4 for details.
Our systematic review included seven studies with a total population of 492 participants, and we included 422 participants in our analysis.
Comparison 1. Dexmedetomidine versus placebo
Six studies (402 participants; Bakhamees 2007; Khanduja 2013; Mohamed 2012; Park 2012; Tufanogullari 2008; Xiao 2013) compared dexmedetomidine with placebo. Most studies reporting data found a reduction in 'rescue' opioid consumption both at three hours after surgery (Bakhamees 2007; Tufanogullari 2008) and at 24 hours after surgery (Bakhamees 2007; Mohamed 2012; Park 2012; Tufanogullari 2008), together with in general no difference in visual analogue scale (VAS) postoperative pain, and the only study (Bakhamees 2007, 80 participants) with an important difference in VAS postoperative pain found a reduction at two hours after surgery in favour of dexmedetomidine. As the result of substantial statistical and clinical heterogeneity, meta‐analyses were not appropriate, and the quality of evidence was very low according to the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) system for our two main outcomes (although low at three hours after surgery for amount of 'rescue' analgesia). Regarding our secondary outcomes,the quantity of data was too small, or the estimates too imprecise or of too poor methodological quality, to enable us to reach any robust conclusions. Regarding post‐interventional complications or side effects, data of low‐quality evidence from one study (Tufanogullari 2008, 80 participants) showed that the number of participants with hypotension requiring intervention was slightly higher in a high‐dose dexmedetomidine group (0.8 µg/kg/h) with a risk ratio of 2.50 (95% confidence interval (CI) 0.94 to 6.66), but groups with lower doses of dexmedetomidine showed no difference compared with control groups.
Comparison 2. Dexmedetomidine versus fentanyl
For the comparison dexmedetomidine versus fentanyl, only one small study (Feld 2006, 20 participants) reported data and found a reduction in both 'rescue' opioid consumption and VAS postoperative pain with dexmedetomidine, but follow‐up was only two hours after surgery, and the amount of evidence must be considered too small to permit a conclusion. No data for our secondary outcomes were reported for this comparison, except for some post‐interventional adverse effects that were too rare and too biased to allow a conclusion regarding differences between groups (need for prolonged mechanical ventilation and hypotension requiring intervention).
No studies compared dexmedetomidine with interventions other than fentanyl or control.
Overall completeness and applicability of evidence
The included studies covered a population not entirely applicable to and representative of the typical population one meets in a hospital setting. The total range of age was limited to 18 to 66 years, and means of age ranged from 29 to 58 years, thus leaving the effect of dexmedetomidine on the older population uninvestigated. Regarding health status, the population represented in the included studies overall was relatively free of co‐morbidity. All studies including American Society of Anesthesiologists Physical Status (ASA) III specifically excluded neurological, cardiovascular, respiratory, renal and hepatic disease. Cultures/countries were restricted to Asia, Egypt and USA. The type of surgery was exclusively elective and showed a preponderance of bariatric surgery, but also included cholecystectomy, abdominal cancer surgery and unspecified abdominal surgery; laparoscopic (≥ 262 participants) and open procedures (≥ 100 participants) were represented.
The interventions explored by included studies consisted of varied dosages and modes of administration (bolus, infusion or both). Studies showed a preponderance of intravenous administration (six studies, 362 participants) compared with other routes (intrathecally in one study with 60 participants).
Outcomes explored by the included studies in general were sufficiently defined and externally applicable, although some valuable details were warranted. For VAS pain, no studies specified whether it occurred at rest or at movement, and only two studies made explicit after what criteria 'rescue' analgesia were administered (Mohamed 2012 at VAS pain ≥ 3, Park 2012 at VAS pain > 4). Tufanogullari 2008 described criteria for the use of patient‐controlled analgesia (PCA) morphine, but not for the other two steps in the three‐step 'rescue' analgesia regimen. Many outcomes of importance for patients were not adequately represented by the included studies, particularly outcomes requiring a longer period of follow‐up.
Quality of the evidence
For an overview of quality of evidence, see Table 1.
According to the GRADE working group (Guyatt 2008; Higgins 2011), we downgraded the quality of evidence according to an evaluation of methodological limitations (internal risk of bias), indirectness of evidence, heterogeneity/inconsistency of results, imprecision of results and publication bias. We upgraded quality of evidence according to any large magnitude of effect, a probable underestimation of effect or a dose‐response gradient. Please note that external validity/applicability is not part of the quality of evidence assessment.
We foundmethodological limitation to be serious for one study (Mohamed 2012). Not only was the risk of selective reporting high as well as the risk of other bias, the precision of results was so accurate, with remarkably small standard deviations, that a far more detailed description of study methods was particularly required. Two other studies (Park 2012; Tufanogullari 2008) had some, less serious, methodological limitations. For more detailed information about internal risk of bias/study limitations, see Figure 2, Figure 3 and Characteristics of included studies.
Regarding indirectness of evidence, we found none among populations, interventions, comparisons nor outcomes. We did not regard bariatric surgery with an obese population (for three out of seven studies) as causing indirectness of evidence, but merely as providing a reason for heterogeneity in results.
Heterogeneity between studies for amount of 'rescue' analgesia after 24 hours was substantial both statistically and clinically. The amount of 'rescue' analgesia seemed very large for Tufanogullari 2008, whereas it seemed surprisingly small for Park 2012 and Mohamed 2012. Possible explanations may include differences in types of surgery and anaesthetic agents; different dosages, timing and route of administration for dexmedetomidine; differences in body mass index of participants and criteria for and mode of administering 'rescue' analgesia (VAS pain ≥ 3 or > 4, frequency of VAS pain evaluation, self administration or administration by personnel), together with differences in the definition of time point noted at 24 hours after surgery or at 'postoperative day one' (which may include up to several additional hours).
Imprecision of results was substantial for Tufanogullari 2008, in particular, considering the large standard deviation for the amount of 'rescue' analgesia; Park 2012 also had considerable imprecision. All studies had a relatively small number of participants. For postoperative nausea and vomiting (PONV), imprecision of results was due to a divergent definition of PONV (all studies reported nausea and vomiting as separate outcomes;therefore, if PONV was reported as one outcome in this review, a unit of analysis error or an underreporting of cases would be committed).
Regarding risk of publication bias, it is difficult to make a proper assessment on this topic because a funnel plot was not considered appropriate. Only one study (Tufanogullari 2008) reported that it was funded in part by a manufacturer of dexmedetomidine, but we did not assess this study as having high risk of bias (see Characteristics of included studies). Studies were all relatively small; therefore a small‐study effect leading to overestimation of effects cannot be ruled out. However, we did not downgrade the quality of evidence but only reported the risk narratively. Please note that publication bias pertaining to outcomes (selective reporting bias) has been evaluated under internal risk of bias (see also Characteristics of included studies).
Consequently, we downgraded the quality of evidence for our two main outcomes by three levels, to very low quality of evidence, signifying that "we are very uncertain about the estimate" (Guyatt 2008). This downgrading was done because of serious study limitations (Mohamed 2012), some study limitations (Park 2012; Tufanogullari 2008), imprecision of results (Park 2012; Tufanogullari 2008) and heterogeneity in effect estimates (Mohamed 2012; Park 2012; Tufanogullari 2008).
Potential biases in the review process
A major problem for this review that may have introduced risk of bias was the great quantity of data that could not be obtained. Six additional studies met the inclusion criteria, except that the type of surgery was mixed with other than abdominal procedures. The number of participants from these studies who underwent an abdominal procedure was at least 87 and was probably around 100. We contacted authors of all of these studies to request individual participant data, without luck (Arain 2004; Bicer 2006; Kilicaslan 2006; Mizrak 2010; Scheinin 1992; Unlugenc 2005). What added to the risk of bias were the studies for which full text could not be retrieved, or for which additional data were needed, that might have met our eligibility criteria. We contacted study authors without luck, or we found that a contact address was not retrievable (Altindis 2008; Anvaroglu 2008; Kordan 2006; Subasi 2012; Yektas 2011). If study authors get back to us at a later stage, we will include the data in future updates of this review.
Another point of potential dispute was that some studies were excluded even though they came close to meeting our eligibility criteria. We had prespecified some time points for some outcomes and were faced with a decision to include or exclude studies with variation in these time points. We made an arbitrary and post hoc decision about how much variation we would permit. An alternative would have been to have no prespecified time points and then combine post hoc what was presented ‐ a choice also not without risk of bias. Among both excluded studies (Harsoor 2014) and included studies, data were not provided for some outcomes (e.g. sedation assessed immediately after surgery (Mohamed 2012), amount of 'rescue' analgesia reported only after 48 hours (Xiao 2013)). Our decision about permissible variation in time points was made before we analysed data properly, thereby reducing risk of bias.
For the outcome of proportion of participants with PONV, three studies reporting data separated PONV into two outcomes (Bakhamees 2007; Mohamed 2012; Tufanogullari 2008). We would have to choose to add the data and risk a unit of analysis error (participants having both nausea and vomiting would count double) or to collect only data for nausea, thereby risking underreporting of the incidence of PONV. We tried to obtain information about whether participants with vomiting also were registered as having nausea, without reply. Anticipating that this probably was the case, we chose to report only data for nausea as PONV. We could have chosen to change our outcome so that 'PONV' would become 'nausea', but as this would be a post hoc decision and as PONV is a generally accepted outcome, this decision was not tempting, although it would have made our reporting precise.
Our decision to use the GRADE system for evaluation of quality of evidence (Guyatt 2008) was made primarily to follow the consensus to apply this tool in Cochrane reviews. However, for this review in which meta‐analyses were not performed, one could point to a weakness by applying this evaluation tool. Speaking of 'low quality of the evidence' and translating it into 'further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate' seems to presume that one pooled and quantifiable estimate can be found. Therefore, for outcomes for which results of studies cannot be pooled in a meta‐analysis, the terminology of the GRADE system may be less than optimal. Our decision to report GRADE Working Group grades of evidence for an outcome if only one study is reporting data can also be discussed because the GRADE system seems to draw its strength primarily as an evaluation tool across studies, inasmuch as the criteria for downgrading the quality of evidence include heterogeneity between studies and publication bias ‐ criteria that cannot be applied unless several studies present data for a particular outcome. Furthermore, when looking at our Table 1, one may find it contra‐intuitive that some of our outcomes, for which the quantity of data was small, have been downgraded by only two levels, whereas other outcomes with a larger quantity of available data have been downgraded by three levels (depending on other qualitative reasons for downgrading). In other terms, the GRADE system does not distinguish between quantity of evidence and quality of evidence but rather incorporates them into one judgement. The GRADE system does not seem to incorporate the possibility of making an 'upper limit' to quality level of evidence when the quantity of data is small. For reviews with scant data, this may result in contra‐intuitive conclusions of the following nature: 'We found low quality evidence for inconclusive evidence'.
For other potential minor biases in the review process, see also Table 5.
4. Potential minor biases in the review process.
| Part of review process | Description of potential minor bias |
| Developing the review question | When choosing the proportion of participants requiring 'rescue' analgesia as one of our secondary outcomes, we overlooked that this outcome might not be meaningful with a design focusing on abdominal surgery. This surgical procedure probably will for almost all participants create some need for 'rescue' analgesia, and hence, a difference between intervention groups regarding this outcome could hardly be detected. After realising this, we could have chosen to exclude this outcome, but this would make a post hoc decision after analysis of results. Had we chosen to exclude this outcome, it would have entailed exclusion of the study by Khanduja 2013, as the study did not report other relevant outcomes for our review |
| Collecting data | One of our included studies was available only in Chinese (Xiao 2013), and because our review was not funded financially, we did not purchase a professional and full translation, but only extraction of data (see Appendix 6) performed on a voluntary basis by a medical expert in native Chinese within The Cochrane Collaboration. Data from tables were extracted by the first and second authors of this review, but regarding assessment of risk of bias, the study was evaluated by only one person (the translator). However, because the study reported very little information about randomization, allocation, blinding, etc, risk of bias introduced in the review process probably was of minor importance. We contacted the Chinese authors for details in English, without reply |
| To keep focus on outcomes with importance for patients, we chose to collect data about heart rate and blood pressure only if they required intervention. Therefore, reports of e.g. significant differences in heart rate or blood pressure have not been collected, unless bradycardia or hypotension requiring intervention was reported. This decision may have led to underreporting of side effects among studies inasmuch as one can imagine that a study reporting a significant difference in heart rate may have omitted data about number of participants when intervention was needed | |
| Presenting results | To make the 'Summary of findings' table as simple as possible, we decided to report only our primary outcomes at the time point 24 hours after surgery. This decision may have represented selective reporting and hence introduced risk of bias, inasmuch as the decision was made after completion of data and analyses. However, all data with all time points were presented in Data and analyses and Table 4 and were also mentioned in several sections |
| Assessing risk of bias | Assessment of 'high risk of other bias' for Tufanogullari 2008 can be debated (see Characteristics of included studies). This is beyond doubt a well‐conducted study with low risk of bias for all other domains, and other reviews have assessed only the study with the highest of quality scores (Blaudszun 2012; Schnabel 2013). We tried to contact study authors to request additional information about the three‐step 'rescue' analgesia regimen, without success |
Agreements and disagreements with other studies or reviews
Two recent systematic reviews on the topic, both of which investigate a broader range of surgical procedures, found results much in accordance with the results of our review.
One systematic review (Schnabel 2013) including 28 randomized controlled trials with 1420 participants investigated the analgesic efficacy and safety of intraoperative dexmedetomidine versus placebo or opioids in adults undergoing surgery (major or minor surgery, comprising gynaecology, ear‐nose‐throat, urology, orthopaedics, neurosurgery, abdominal surgery, heart surgery). The median quality score of data reporting was 5 (range 1 to 7) on a modified 7‐point Oxford scale. This meta‐analysis also looked at the same primary outcomes as this review, at time points of one, two, four, 24 and 48 hours after surgery. Meta‐analyses for these outcomes were appropriate only for the comparison of dexmedetomidine versus placebo. Postoperative pain (numerical rating scale (NRS) 0 to 10) was significantly reduced in the dexmedetomidine group versus the placebo group (including nine studies and 492 participants) at all time points, with a maximum mean difference of ‐1.59 (95% CI ‐2.37 to ‐0.8) at one hour after surgery, showing diminishing differences with time, with a minimum mean difference of ‐0.41 (‐0.53 to ‐0.29) at 48 hours after surgery. Postoperative morphine consumption (including 10 studies with 528 participants) was significantly reduced in the dexmedetomidine group compared with the placebo group, with mean differences increasing from ‐3.79 mg (95% CI ‐5.36 to ‐2.23) to ‐17.24 mg (95% CI ‐24.38 to ‐10.10) to ‐39.22 mg (95% CI ‐47.52 to ‐30.91) at time points two, 24 and 48 hours after surgery. We found a non‐significant reduction in the remaining time points at one and four hours after surgery. Heterogeneity was substantial (I2 statistic > 90%), however, for most of the time points for both outcomes. We performed several subgroup analyses, including analyses for different types of surgery, and found that for abdominal surgery, the reduction in NRS pain was smaller than for other types of surgery, whereas the significant reduction in morphine consumption was not significantly different compared with other surgical procedures. Regarding secondary outcomes, participants treated with dexmedetomidine had a non‐significant reduction in risk ratio for PONV at most time points. The review also found a significant reduction in pruritus for dexmedetomidine treated groups, but for other opioid‐related side effects, such as urinary retention and respiratory depression, data were insufficient. Intraoperative bradycardia requiring intervention (e.g. atropine) was significantly greater in participants treated with dexmedetomidine than for those given placebo (risk ratio (RR) 2.66, 95% CI 1.54 to 4.58), whereas for intraoperative hypotension, the risk ratio was not significantly higher in the dexmedetomidine treated groups (RR 1.85, 95% CI 0.83 to 4.13).
The other systematic review and meta‐analysis (Blaudszun 2012) investigated randomized trials conducted to test any systemic alpha‐2 agonist (vs placebo or no treatment) administered perioperatively to adults undergoing non‐cardiac surgery under general anaesthesia. Inclusion criteria consisted of reported data on postoperative cumulative opioid consumption or pain intensity. Review authors included 30 studies with 1792 participants, in which clonidine (19 studies) or dexmedetomidine (11 studies) was compared with placebo (no head‐to‐head comparison of clonidine and dexmedetomidine). Types of surgery included abdominal (14 studies), hysterectomy (five), spine (four), ear–nose–throat (one), orthopaedic (one), vascular (one) and not specified (four). The median quality score was 4 (range 2 to 7) on a modified 7‐point Oxford scale. This review found a morphine‐sparing effect at 24 hours after surgery (four studies, 419 participants) with a mean difference of ‐14.5 mg (95% CI ‐22.1 to ‐6.8) for dexmedetomidine (which was greater than for clonidine at ‐4.1 mg (95% CI ‐6.0 to‐2.2), although heterogeneity was considerable (I2 statistic = 91%). For VAS postoperative pain (0 to 10 cm) at 24 hours after surgery (three studies, 225 participants), a significant reduction in mean difference (‐0.6 cm, 95% CI ‐0.9 to ‐0.2; I2 statistic = 33%) favoured dexmedetomidine versus placebo (approximately the same for clonidine). At 48 hours after surgery, both alpha‐2 agonists had lost their analgesic effects. The incidence of early nausea was decreased with both drugs compared with control. Only three studies reported haemodynamic side effects for dexmedetomidine, showing increased risk of postoperative bradycardia, whereas the quantity of reported data about adverse effects for clonidine was larger and showed increased risk of intraoperative and postoperative hypotension. The review authors suspected a possible element of selective reporting in the dexmedetomidine trials concerning haemodynamic adverse effects.
It is worth mentioning the results of two of the studies awaiting classification, for which the main portion of participants underwent abdominal surgery. Both trials were included in the two reviews mentioned above. The first study (Unlugenc 2005) found results much in accordance with the results of our review, whereas the other study (Arain 2004) found an opioid‐sparing effect of dexmedetomidine only during the first few hours after surgery.
Unlugenc 2005, a Turkish randomized controlled trial, investigated the analgesic effect of dexmedetomidine for 60 adults (18 to 64 years of age) undergoing abdominal surgery (mini‐laparotomy (three); cholecystectomy (30); splenectomy (four); inguinal, incisional or umbilical hernia repair (23)) who were classified as ASA I to II and were treated with an intravenous bolus of dexmedetomidine 1 µg/kg or placebo at 10 minutes before induction of anaesthesia. This study found a significant reduction in 'rescue' morphine consumption in favour of dexmedetomidine at time points six, 12 and 24 hours after surgery. At 24 hours after surgery, the dexmedetomidine group had received a median of PCA morphine (mg) of 23.8 (range 69.5) versus a median of 44 (range 52) in the placebo group (P value < 0.01) ‐ a 28% reduction. Review authors reported no significant differences between groups in VAS pain at rest. The study found no significant differences between groups for sedation score (5 point scale), nausea score (number of participants with nausea not reported), time to extubation nor time to recovery. Opioid‐induced side effects (urinary retention or pruritus) were very few and showed no significant differences between groups. See Characteristics of studies awaiting classification. We would evaluate risk of bias for this study as low for all domains.
The other study, Arain 2004, a randomized controlled trial from USA, investigated the effect of dexmedetomidine (bolus 1 µg/kg and infusion 0.4 µg/kg/h, initiated 30 minutes before end of surgery) versus morphine in 34 adults (ASA I to III, mean age 60 years) undergoing intra‐abdominal (15), major orthopaedic (13) and other surgery (six). The study found no difference in the use of PCA morphine (based on frequent increments and no total) during 24 hours after stay in the post‐anaesthesia care unit (PACU), but a significant difference in the total amount of 'rescue' morphine administered by a nurse in the PACU (precise length of stay not reported) favouring dexmedetomidine (mean difference ‐4.50 mg, 95% CI ‐5.41 to ‐3.59), together with a significant reduction in the number of participants needing 'rescue' analgesia during the first 60 minutes after surgery (RR 0.07, 95% CI 0.01 to 0.43). This study found no significant differences between groups for VAS postoperative pain, sedation or nausea (follow‐up for these outcomes was only 100 minutes after surgery). Investigators reported no cardiovascular or respiratory complications requiring intervention (heart rate in PACU was significantly lower in the dexmedetomidine group; mean arterial blood pressure showed no significant differences between groups, except for a small and transient increase in mean arterial blood pressure after bolus of dexmedetomidine). We evaluated the study to have low risk of bias in most domains and no high risk of bias. See Characteristics of studies awaiting classification for additional details.
When we look at randomized trials (including reviews of randomized trials), side effects and complications (or protection from these) associated with the use of dexmedetomidine are difficult to detect, typically because they are underpowered. A large systematic review and meta‐analysis (Lin 2012) investigated the safety and sedative effects of dexmedetomidine compared with placebo or another sedative agent in elective cardiac surgery patients, with administration of dexmedetomidine that was not perioperative but was provided for postoperative sedation for at least six hours. This review also included studies other than randomized controlled trials (retrospective and prospective cohort studies), and the number of participants amounted to 16,818 (11 studies). The review found that dexmedetomidine was associated with shorter length of mechanical ventilation and lower risk of delirium, ventricular tachycardia and hyperglycaemia, but that the risk of bradycardia may be increased. Review authors also concluded that dexmedetomidine may not increase the risk of hypotension, atrial fibrillation, postoperative nausea and vomiting, reintubation within five days, cardiovascular complications, postoperative infection or hospital mortality. Additionally, they found no significant differences in length of intensive care unit (ICU) or hospital stay. An interesting observation was that the review found no significant differences in morphine equivalents (mean difference 0.45, 95% CI ‐1.86 to 2.77; P value = 0.70) between the included studies, which described no additional details. Although this observation was made in a very different setting and in another population than was included in our eligibility criteria, it disagrees with findings of our review and of the above‐mentioned reviews.
Authors' conclusions
Implications for practice.
The findings of our systematic review suggest that dexmedetomidine, compared with placebo, seems to have analgesic and opioid‐sparing effects, but when the comparison is made with placebo, this is hardly surprising. The analgesic effects seemed to last up to 24 hours after surgery, even though half of the dexmedetomidine is eliminated from the organism within two hours. Pooled quantification of the opioid‐sparing effect was not possible in this review, but the opioid‐sparing effect was around or above 25% for most studies during 24 hours after surgery, without a significant difference in postoperative pain. For our main outcomes, the quality of the evidence was very low according to the GRADE system, signifying that "we are very uncertain about the estimate" (Guyatt 2008). We cannot rule out a small‐studies effect with overestimation of treatment effects and underreporting of relevant side effects. Applicability (external validity) of the results was limited to middle‐aged participants with a minimum of co‐morbidity when the reason for abdominal surgery was not considered, and a relative overweight of bariatric surgery and thus adipose participants was noted.
Our primary outcomes cannot be separated from one another in the evaluation of pain and treatment of pain, but what matters to patients is that they experience a minimum of pain, and in this regard, a presumed opioid‐sparing effect of a treatment in itself is not important to patients. What is more essential involves a reduction in opioid‐related side effects, improved postoperative recovery and a minimum of other side effects and complications. With selection of our secondary outcomes (postoperative sedation, postoperative nausea and vomiting, gastrointestinal function and mobilization), we had hoped to be able to evaluate some of these important issues for patients, but the included studies provided insufficient data. With practical use of dexmedetomidine, one should still be prepared that side effects, particularly hypotension and bradycardia, could require intervention. The optimal dosage, timing and route of administration in the perioperative use of dexmedetomidine have not been settled.
A comparison of dexmedetomidine with other analgesic agents has not been possible, as only one of our included studies (20 participants) provided data, with fentanyl as a comparison.
Implications for research.
One great limitation of this systematic review was that our choice to exclude surgery other than abdominal entailed that a great deal of available data could not be included because they were derived from studies with mixed surgery (or unspecified), and without the opportunity to obtain individual participant data. It is not in our ability to judge whether it can be recommended that future research should be more restricted to specific types of surgery, but it is obvious that future updates of this review would benefit from more research restricted to abdominal surgery. Furthermore, it is likely that studies that investigate mixed surgery, comprising for example minor or superficial surgery together with abdominal surgery, may be unlikely to contribute to patient‐important outcomes and side effects that are particularly relevant in abdominal surgery, such as paralytic ileus, time to mobilization or urinary retention.
Concerning abdominal surgery, a more exact quantification of the opioid‐sparing effects of dexmedetomidine would of course be relevant for comparison with other analgesic treatments, but this should not be a stand‐alone aim of future research. Future investigators must focus on patient‐important issues, including outcomes with a longer period of follow‐up. Secondary outcomes of this review should not be considered as a gold standard check list for future research, but merely as examples of relevant outcomes to patients. One could think of many other highly important outcomes, such as postoperative infection, rehospitalization, delirium, patient satisfaction, chronic postoperative pain and of course mortality. Larger studies are needed to detect such outcomes.
To pool data in reviews and meta‐analyses and compare study results, it is important to seek consensus concerning evaluation methods and parameters for outcomes. Areas for possible improvement include evaluation of sedation, the distinction between VAS pain at rest and at movement, a systematic reporting of baseline data for evaluated outcomes and a consensus to report postoperative nausea and vomiting as primarily one outcome ‐ PONV ‐ not separately as nausea and vomiting (because one cannot know if some participants have both and thereby will count double in pooling of data, thus causing a unit of analysis error with a meta‐analysis). Furthermore, a report of only VAS pain should never be provided without a report of amount of 'rescue' analgesia and vice versa.
A challenge in performing meta‐analyses and reviews of postoperative pain treatment is the great diversity among 'rescue' analgesia regimens. Reports of the criteria for administering 'rescue' analgesia provided together with the frequency of evaluation of pain are relevant data for a systematic review.
Concerning the methodological quality of studies, the description of random sequence generation, details of blinding methods, allocation concealment methods, etc, should be improved. Without this detailed information, the judgement 'unclear risk of bias' may cover studies that should not have been downgraded and studies that should have been even further downgraded.
The optimal dosage, timing and route of administration for the perioperative use of dexmedetomidine in abdominal surgery remain to be clarified to show a minimum of harms and a maximum of benefit. Comparisons of dexmedetomidine with other analgesic agents are as yet sparse.
Acknowledgements
We thank Jane Cracknell for her help as Managing Editor of the Cochrane Anaesthesia, Critical and Emergency CARE Group (ACE). We thank Karen Hovhannisyan (Trials Search Co‐ordinator) for help with the search strategy. We would like to thank Michael Bennett (Content Editor); Cathal Walsh (Statistical Editor); Chi Wai Cheung, Stephan Schug, Fang Gao Smith and Francis Bonnet (Peer Reviewers); Nathan Pace (Co‐ordinating Editor), Toby Lasserson (Senior Editor, Cochrane Editorial Unit) and Anne Lyddiatt (Consumer Referee) for help and editorial advice provided during preparation of this systematic review. In addition, we wish to thank Asbjørn Hróbjartsson for methodological advice provided during the protocol process, and Ismael Gögenur and Henrik Jørgensen for advice given on selection of relevant outcome measures. We would also like to thank Rao Sun for helping with extraction of data from a Chinese article, and Karen Hovhannisyan for helping with extraction of data from a Russian article.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Dexmedetomidine] explode all trees #2 MeSH descriptor: [Adrenergic alpha‐Agonists] explode all trees #3 (Precedex or Dexmedetomidin*) or ((adren?ergic or alpha) near agonist*) #4 #1 or #2 or #3 #5 MeSH descriptor: [Pain, Postoperative] this term only #6 MeSH descriptor: [Acute Pain] explode all trees #7 MeSH descriptor: [Postoperative Period] this term only #8 (acute near pain):ti,ab or (pain near measur*) or (recovery near (operat* or surgery)) or post?an?esthetic care unit or PACU or (gastrointestinal near function):ti,ab or (opioid* near sparing near effect) #9 #5 or #6 or #7 or #8 #10 #4 and #9
Appendix 2. MEDLINE search strategy (Ovid SP)
1 exp Dexmedetomidine/ or Adrenergic alpha‐Agonists/ or (Precedex or Dexmedetomidin*).af. or ((adren?ergic or alpha) adj3 agonist*).mp. 2 Pain, Postoperative/ or exp Acute pain/ or Postoperative Period/ or (acute adj3 pain).ti,ab. or (pain adj4 measur*).mp. or (recovery adj3 (operat* or surgery)).mp. or post?an?esthetic care unit.mp. or PACU.mp. or (gastrointestinal adj2 function).ti,ab. or (opioid* adj2 sparing adj2 effect).mp. 3 1 and 2 4 ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or drug therapy.fs. or randomly.ab. or trial.ab. or groups.ab.) not (animals not (humans and animals)).sh. 5 3 and 4
Appendix 3. EMBASE (Ovid SP) search strategy
1. exp dexmedetomidine/ or alpha adrenergic receptor stimulating agent/ or (Precedex or Dexmedetomidin*).af. or ((adren?ergic or alpha) adj3 agonist*).mp. 2. postoperative pain/ or pain/ or postoperative period/ or (acute adj3 pain).ti,ab. or (pain adj4 measur*).mp. or (recovery adj3 (operat* or surgery)).mp. or post?an?esthetic care unit.mp. or PACU.mp. or (gastrointestinal adj2 function).ti,ab. or (opioid* adj2 sparing adj2 effect).mp. 3. 1 and 2 4. Randomized controlled trial/ or Controlled study/ or Randomization/ or Double blind procedure/ or Single blind procedure/ or Clinical trial/ or (clinical adj5 trial$).ti,ab,hw. or ((doubl$ or singl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).ti,ab,hw. or Placebo/ or Placebo$.ti,ab,hw. or Random$.ti,ab,hw. or Methodology.sh. or latin square.ti,ab,hw. or crossover.ti,ab,hw. or cross‐over.ti,ab,hw. or Crossover Procedure/ or Drug comparison/ or Comparative study/ or (comparative adj5 trial$).ti,ab,hw. or (control$ or prospectiv$ or volunteer$).ti,ab,hw. or exp "Evaluation and Follow Up"/ or Prospective study/ 5. 3 and 4
Appendix 4. ISI Web of Science search strategy
#1 TS=(Precedex or Dexmedetomidin*) or TS=((adren?ergic or alpha) SAME agonist*) #2 TS=((acute SAME pain) or (pain SAME measur*) or (recovery SAME (operat* or surgery)) or post?an?esthetic care unit or PACU or (gastrointestinal SAME function) or (opioid* adj2 sparing adj2 effect)) #3 #1 and #2 #4 TS=(random* or ((clinical or controlled) SAME trial*) or placebo* or multicenter* or prospective) or TS=((blind* or mask*) SAME (single or double or triple or treble)) #5 #3 and #4
Appendix 5. CINAHL (EBSCOhost) search strategy
S1 ( MH "Adrenergic Alpha‐Agonists") OR ( Precedex or Dexmedetomidin* ) OR ( ((adren?ergic or alpha) N3 agonist*) ) S2 ( (MH "Postoperative Pain") OR (MM "Acute Pain (Saba CCC)") OR (MH "Postoperative Period") ) OR ( (acute N3 pain) or (pain N4 measur*) or (recovery N3 (operat* or surgery)) or post?an?esthetic care unit or PACU or (gastrointestinal N2 function) or (opioid* adj2 sparing adj2 effect) ) S3 S1 and S2
Appendix 6. Data collection form
| Instructions: |
|
| Review title and ID |
| CARG 260, Dexmedetomidine for postoperative pain in adults |
| Date form completed (e.g. 3 Nov 2011) |
| Name/ID of person extracting data |
| Study ID (surname of first author and year first full report of study was published e.g. Smith 2001) |
| Report IDs of other reports of this study (e.g. duplicate publications, follow‐up studies) |
| Report title |
| Reference details and report ID (including journal, authors, department, etc) |
| Report author contact details |
| Publication type (underscore) |
| full report abstract letter other |
| Important notes |
| |
| Data that have not yet been extracted |
| Questions/doubts (include location in text) |
| |
1. Study eligibility
| Type of study: randomized controlled trial, including factorial trial and cluster‐randomized, but not cross‐over, design (Y/N/UN) |
| Participants: adults undergoing abdominal surgery, open or laparoscopic (Y/N/UN) |
| Type of intervention: dexmedetomidine administered perioperatively and as the sole difference between the intervention group and the control group(s), or administered perioperatively as part of a factorial trial (Y/N/UN) |
| At least one of the following outcomes (underscore) |
| Dose of rescue opioid Postoperative pain measured by VASa Proportion of participants needing rescue analgesia Time to first rescue analgesia Postoperative sedation Proportion of participants with nausea/vomiting or needing antiemetics Time to first flatus/stool or proportion of participants with delay to first flatus/stool Time to out‐of‐bed mobilization or proportion of participants with delay to out‐of‐bed mobilization |
| INCLUDE only if yes in all 4 categories (Y/N/UN) |
| EXCLUDE and record here the information to be inserted into ‘Table of excluded studies’ |
| Notes |
aVAS = visual analogue scale.
DO NOT PROCEED IF STUDY EXCLUDED FROM REVIEW
2. Characteristics of study
| Setting (underscore) |
| Single‐centre Multi‐centre |
| Inclusion criteria |
| Exclusion criteria |
| Informed consent obtained |
| Ethical approval (underscore) |
| obtained not needed not obtained though needed not reported |
| Aim of study (you may copy and paste from the ‘Objectives’ section) |
| Design of study (underscore) |
| 2 parallel groups multi‐arm parallel groups cluster‐randomized factorial trial (state 2 × 2, 2 × 3, etc) |
| Intervention group(s) vs control group(s) (e.g. dexmedetomidine vs morphine plus diclofenac vs placebo) |
| Outcomes measured (list all outcomes mentioned in the report even if no results reported) |
| Outcomes reported (list all outcomes when corresponding results are reported in text or in tables/figures) |
|
In tables/figures:
In text: Qualitatively: Quantitatively: Outcomes measured, but not reported: |
| Total duration of study |
| Notes |
3. Participants (provide overall data and, if available, comparative data for each intervention or comparison group. Use group code name, e.g. ‘women D:11, M:7’)
| List code names for all groups (e.g. ‘D = dexmedetomidine group, M = morphine group’) |
| Total number randomized, with group proportions (e.g. ‘40, 18:22’) |
| Baseline imbalances (describe) |
| Prespecified criteria for exclusion of participants after randomization |
| Withdrawals/exclusions and reasons why (if not described under results) |
| Age (describe distribution of age as reported) |
| Sex (numbers of men/women) |
| Weight and height |
| Country |
| Other relevant sociodemographics |
| Description of surgical procedure(s) (and underscore elective/acute) |
| Elective Acute Description |
| Anaesthetic method (UA = universal anaesthesia, RA = regional anaesthesia) |
| Duration of surgery |
| American Society of Anesthesiologists (ASA) Physical Status |
| Other relevant treatment received (state if before, under or after surgery) |
| Notes |
4. Subgroups
| Subgroup analyses performed in paper, if any | ||
| Subgroups prespecified for review (fill in number of participants, if information available in paper) | ||
| Laparoscopic procedure | Open procedure | Information not available in paper (NA) |
| Route of administration 1 | Route of administration 2 | NA |
| Bolus only | Infusion (with or without loading dose) | NA |
| Notes | ||
5. Intervention groups (copy and paste this section for each intervention and comparison group)
| Group name (including code) |
| Number randomized to group |
| Description (including content, dose(s), etc) |
| Timing (time point relative to surgery, frequency, duration of each episode) |
| Delivery (mechanism, medium) |
| Notes |
6. Outcomes (copy and paste this section for each outcome)
| Outcome name |
| Time points when measurements were taken during the study |
| Time points reported in the study |
| Time points you are using in RevMan 5 |
| Outcome definition |
| Unit of measurement |
| For measurement scales: state which score is best (underscore) and state name of scale with descriptions |
| Highest Lowest Name of scale Description of steps |
| Notes |
7. Results (copy and paste the appropriate section for each outcome, including additional tables for each time point and subgroup as required)
7.a. Dichotomous outcome
| Comparison | |||
| Outcome | |||
| Subgroup (if relevant) | |||
| Time point (specify time point 0) (if relevant) | |||
| Intervention | Number of events | Number of participants | Number of missing participants and reasons |
| Comparison | Number of events | Number of participants | Number of missing participants and reasons |
| Source of data (pg., fig., table, from contact with study author) | |||
| Results calculated by you? (state formula if yes) | |||
| Results estimated from graphs? (state if yes) | |||
| Unit of analysis (state if body part and delete 'individuals') | |||
| Individuals | |||
| Notes | |||
7.b. Continuous outcome
| Comparison | |||
| Outcome | |||
| Subgroup (if relevant) | |||
| Time point (specify time point 0) (if relevant) | |||
| Intervention | |||
|
Mean (or other measure) |
SD (or other variance) | Number of participants | Number of missing participants and reasons |
| Comparison | |||
| Mean (or other measure) | SD (or other variance) | Number of participants | Number of missing participants and reasons |
| Source of data (pg., fig., table, from contact with study author) | |||
| Results calculated by you? (state formula if yes) | |||
| Results estimated from graphs? (state if yes) | |||
| Unit of analysis (state if body part and delete ‘individuals’) | |||
| Individuals | |||
| Notes | |||
7.c. Other outcome
| Comparison | |||
| Outcome | |||
| Unit of measurement | |||
| Subgroup (if relevant) | |||
| Time point (specify time point 0) | |||
| Intervention | |||
|
Mean (or other measure) |
SD (or other variance) | Number of participants | Number of missing participants and reasons |
| Comparison | |||
| Mean (or other measure) | SD (or other variance) | Number of participants | Number of missing participants and reasons |
| Source of data (pg., fig., table, from contact with study author) | |||
| Results calculated by you? (state formula if yes) | |||
| Results estimated from graphs? (state if yes) | |||
| Unit of analysis (state if body part and delete ‘individuals’) | |||
| Individuals | |||
| Notes | |||
7.d. Outcome reported in a narrative manner
| Comparison |
| Outcome |
| Subgroup (if relevant) |
| Results (describe) |
| Source of data (pg., table, from contact with study author) |
| Notes |
8. Other information
| Study funding sources/University |
| Possible conflicts of interest |
| Key conclusions of study authors (if relevant) |
| References or contact details for other potentially eligible trials not already identified (published or unpublished) |
| Correspondence required for further study information (from whom, about what and when) |
Data and analyses
Comparison 1. Dexmedetomidine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Amount of 'rescue' opioid 3 hours after surgery (intravenous morphine equivalents) | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Amount of 'rescue' opioid 24 hours after surgery (intravenous morphine equivalents) | 4 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Postoperative pain 3 hours after surgery (VAS 0‐100) | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Postoperative pain 6 hours after surgery (VAS 0‐100) | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5 Postoperative pain 12 hours after surgery (VAS 0‐100) | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6 Postoperative pain 24 hours after surgery (VAS 0‐100) | 4 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7 Postoperative nausea and vomiting (PONV) | 3 | Risk Ratio (IV, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bakhamees 2007.
| Methods | RCT Two parallel groups |
|
| Participants |
Number: 80 (for gender, see notes) Country: Egypt Surgery: laparoscopic Roux‐en‐Y gastric bypass, general anaesthesia, elective ASA: II or III Age: 26 to 55 years Other Inclusion criteria: morbidly obese Exclusion criteria: clinically significant brain, cardiac, respiratory or liver disease |
|
| Interventions |
vs
All participants:
|
|
| Outcomes | VAS pain 0 to 10 at 2 hours PO Amount of PCA morphine at 2 hours and 1 day PO Number of participants with nausea Number of participants with vomiting |
|
| Notes | Total amount of intraoperative propofol (mg, mean ± SD) was lower in the dexmedetomidine group (1447 ± 310) than in the placebo group (2162 ± 454) Total amount of intraoperative fentanyl (μg ± SD) was lower in the dexmedetomidine group (199.4 ± 44.6) than in the placebo group (362.2 ± 57.2) Study authors contacted for additional information about random sequence generation, allocation concealment, additional blinding details, gender of participants, etc, without reply The article contained an error in report of gender, stating number of men as 34 and women as 26, leaving the gender of 20 participants unspecified |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "patients...were randomly assigned" Comment: nothing reported about how randomization was made |
| Allocation concealment (selection bias) | Unclear risk | Nothing reported |
| Blinding of participants and personnel (performance bias) Pain, 'rescue' analgesia, postoperative sedation | Low risk |
Quote: "Patients and investigators recording data in the operating room were blinded to the treatment..., but the anaesthesiologist was aware of the treatment condition. The same surgeon performed all of the surgeries" Comment: No blinding of the anaesthesiologist may have influenced the administered amount of intraoperative propofol and fentanyl, which was higher in the placebo group for both drugs. Particularly fentanyl could have an influence on the postoperative need for PCA morphine. Taking the direction of this bias into account, the actual measured effect was an underestimate, and therefore the risk of bias was low. Participants were blinded, and this was essential for a low risk of bias concerning PCA morphine |
| Blinding of participants and personnel (performance bias) PONV | Unclear risk |
Quote: same as above Comment: same as comment above ‐ not only fentanyl but also propofol could have an influence on PONV. In this case, however, the direction of bias was not clear |
| Blinding of participants and personnel (performance bias) Time to first passage of flatus or stool | Unclear risk | Not relevant |
| Blinding of participants and personnel (performance bias) Time to first out‐of‐bed mobilization | Unclear risk | Not relevant |
| Blinding of participants and personnel (performance bias) Post‐interventional complications or side effects | Unclear risk | Not relevant |
| Blinding of outcome assessment (detection bias) Pain, 'rescue' analgesia, postoperative sedation | Low risk |
Quote: "investigators recording data in the operating room were blinded to the treatment" Quote: "pain scores were obtained...by a nurse who was blinded to the treatment procedure" Comment: probably done. Amount of PCA morphine was probably assessed by the same nurse who was assessing pain scores |
| Blinding of outcome assessment (detection bias) PONV | Unclear risk |
Quote: "investigators recording data in the operating room were blinded to the treatment" Quote: "pain scores were obtained...by a nurse who was blinded to the treatment procedure" Comment: not explicitly stated for PONV |
| Blinding of outcome assessment (detection bias) Time to first passage of flatus or stool | Unclear risk | Not relevant |
| Blinding of outcome assessment (detection bias) Time to first out‐of‐bed mobilization | Unclear risk | Not relevant |
| Blinding of outcome assessment (detection bias) Post‐interventional complications or side effects | Unclear risk | Not relevant |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data |
| Selective reporting (reporting bias) | Low risk | Comment: No protocol available, but all outcomes mentioned in Methods section reported quantitatively and for all prespecified time points |
| Other bias | Low risk | No other apparent bias |
Feld 2006.
| Methods | RCT, 2 parallel groups | |
| Participants |
Number: 20 men (n = 7) and women (n = 13) Country: USA Surgery: open gastric bypass, general anaesthesia, elective ASA: II or III Age: 26 to 55 years Other inclusion criteria: bariatric patients Exclusion criteria: pregnancy, clinically significant brain, cardiac, respiratory or liver disease |
|
| Interventions |
vs
All participants:
|
|
| Outcomes | VAS pain at 2 hours PO, reported as median and range Amount of PCA morphine at 2 hours PO Post‐interventional complications reported post hoc: number of participants with hypotension requiring intervention and number of participants requiring prolonged mechanical ventilation |
|
| Notes | Less thiopental (mg/kg, mean ± SD) was used for induction of anaesthesia in the dexmedetomidine group (2.1 ± 0.5) compared with the fentanyl group (3.1 ± 0.6) The dexmedetomidine group required less desflurane concentration compared with the fentanyl group to maintain the target BIS level Time of follow‐up was 2 hours PO Baseline characteristics concerning height, weight and body mass index probably reported with error. Study authors were contacted for this additional information, including details of allocation concealment, without luck |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "patients were randomized using a computer‐generated random number table" Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | Nothing reported |
| Blinding of participants and personnel (performance bias) Pain, 'rescue' analgesia, postoperative sedation | Low risk |
Quote: "Patients and investigators recording data in the operating room were blinded to the treatment..., but the anaesthesiologist was aware of the treatment condition. The same surgeon performed all of the surgeries" Comment: incomplete blinding, but unlikely to have an effect on the need for rescue analgesia |
| Blinding of participants and personnel (performance bias) PONV | Unclear risk | Not relevant |
| Blinding of participants and personnel (performance bias) Time to first passage of flatus or stool | Unclear risk | Not relevant |
| Blinding of participants and personnel (performance bias) Time to first out‐of‐bed mobilization | Unclear risk | Not relevant |
| Blinding of participants and personnel (performance bias) Post‐interventional complications or side effects | High risk |
Quote: "Patients and investigators recording data in the operating room were blinded to the treatment..., but the anaesthesiologist was aware of the treatment condition. The same surgeon performed all of the surgeries" Comment: No blinding of the anaesthesiologist may have influenced the administered amount of intraoperative desflurane, which was higher in the fentanyl group. This may have influenced post‐interventional complications, in particular circulatory complications for which no report provided prespecified criteria for giving epinephrine |
| Blinding of outcome assessment (detection bias) Pain, 'rescue' analgesia, postoperative sedation | Low risk |
Quote: "investigators recording data in the operating room were blinded to the treatment" Quote: "VAS pain, heart rate and blood pressure were recorded postoperatively by a nurse blinded to the treatment procedure" Comment: Amount of PCA morphine probably was assessed by the same nurse who was assessing pain scores |
| Blinding of outcome assessment (detection bias) PONV | Unclear risk | Not relevant |
| Blinding of outcome assessment (detection bias) Time to first passage of flatus or stool | Unclear risk | Not relevant |
| Blinding of outcome assessment (detection bias) Time to first out‐of‐bed mobilization | Unclear risk | Not relevant |
| Blinding of outcome assessment (detection bias) Post‐interventional complications or side effects | High risk |
Quote: "Patients and investigators recording data in the operating room were blinded to the treatment..., but the anaesthesiologist was aware of the treatment condition. The same surgeon performed all of the surgeries" Quote: "VAS pain, heart rate and blood pressure were recorded postoperatively by a nurse blinded to the treatment procedure" Comment: The non‐blinded anaesthesiologist was likely to have had a role in assessment and treatment of circulatory and respiratory complications |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data |
| Selective reporting (reporting bias) | High risk | No protocol available. Regarding the post‐interventional complications reported post hoc, prolonged mechanical ventilation could not have been foreseen, but hypotension requiring intervention (epinephrine 25 mg intramuscularly) could have been prespecified, and the criteria for giving epinephrine should have been prespecified |
| Other bias | Low risk | No other apparent bias |
Khanduja 2013.
| Methods | RCT, single‐blinded, 2 parallel groups | |
| Participants |
Number: 60 men (n = 12) and women (n = 48) Country: India Surgery: laparoscopic cholecystectomy, general anaesthesia, elective ASA: not reported, probably I and II (judged by exclusion criteria) Age: 20 to 65 years Other inclusion criteria: none reported Exclusion criteria: anaemia, long‐term medications or any medication within 1 week before surgery, history of any chronic disease, cardiac problem, history of drug abuse, consumption of more than 30 g alcohol/d, use of β‐blockers and abnormal preoperative electrolyte concentrations |
|
| Interventions |
vs
All participants:
|
|
| Outcomes | Number of participants with postoperative pain. No time point specified, but probably immediately after extubation | |
| Notes | A significant difference was reported in the total amount of administered pentazocine (mg, mean, SD): 17.9 ± 4.13 in the dexmedetomidine group vs 29.4 ± 4.272 in the placebo group (P value < 0.001), including intraoperative need and predetermined difference in induction dose Duration of study ≥ 30 minutes after surgery, not further specified. Study authors contacted for details of follow‐up, random sequence generation, allocation concealment, etc, without luck |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "...randomized..." Comment: not reported how |
| Allocation concealment (selection bias) | Unclear risk | Nothing reported |
| Blinding of participants and personnel (performance bias) Pain, 'rescue' analgesia, postoperative sedation | Low risk |
Quote: " ...single blinded..." Comment: probably non‐blinded anaesthesiologist assessing all outcomes (time of follow‐up approximately 30 minutes after surgery). This could have influenced the intraoperatively administered amount of pentazocine, which was significantly different between groups. An unblinded anaesthesiologist might have an interest in showing reduced need for intraoperative analgesia/anaesthesia in the intervention group, or he might anticipate that participants would have more pain. Thus, the possible direction of bias would be to underestimate the effect of intervention; therefore the risk of bias was low |
| Blinding of participants and personnel (performance bias) PONV | Unclear risk | Not relevant |
| Blinding of participants and personnel (performance bias) Time to first passage of flatus or stool | Unclear risk | Not relevant |
| Blinding of participants and personnel (performance bias) Time to first out‐of‐bed mobilization | Unclear risk | Not relevant |
| Blinding of participants and personnel (performance bias) Post‐interventional complications or side effects | Unclear risk | Not relevant |
| Blinding of outcome assessment (detection bias) Pain, 'rescue' analgesia, postoperative sedation | Low risk |
Quote: "...single blinded..." Comment: the same comment as under performance bias |
| Blinding of outcome assessment (detection bias) PONV | Unclear risk | Not relevant |
| Blinding of outcome assessment (detection bias) Time to first passage of flatus or stool | Unclear risk | Not relevant |
| Blinding of outcome assessment (detection bias) Time to first out‐of‐bed mobilization | Unclear risk | Not relevant |
| Blinding of outcome assessment (detection bias) Post‐interventional complications or side effects | Unclear risk | Not relevant |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to allow a judgement; no protocol available |
| Other bias | Unclear risk | Insufficient information to allow a clear judgement |
Mohamed 2012.
| Methods | RCT, 3 parallel groups | |
| Participants |
Number: 90 men (n = 30) and women (n = 60) Country: Egypt Surgery: major abdominal cancer surgery, general anaesthesia, elective Age: 25 to 55 years ASA: I or II Other inclusion criteria: weight 50 to 85 kg Exclusion criteria: allergy, bleeding diathesis, liver/renal dysfunction, drug/alcohol abuse, psychiatric illness interfering with pain assessment |
|
| Interventions |
vs
vs
All participants:
|
|
| Outcomes | Total analgesic consumption during 24 hours (intravenous tramadol 100 mg when VAS ≥ 3 or on participant's request) VAS pain at PACU arrival and at 2, 4, 6, 8, 12 and 24 hours PO Time to first request of analgesia Number of participants with nausea during 24 hours PO Number of participants with vomiting during 24 hours PO |
|
| Notes | Nausea and vomiting are reported as 2 separate outcomes Postoperative sedation score was measured with 5‐scale Observer's Assessment of Alertness/Sedation (OAA/S) scale, but with no specified time points and nothing else reported than "no significance between groups" Please note that ASA was judged to be I, even if participants were known to have abdominal cancer No information about specific type of surgery (which type of cancer, laparoscopic vs open) Study authors contacted about the above mentioned and details of relevance for risk of bias assessment, without reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "this randomized trial..." Quote: "patients were allocated to one of 3 groups..." Comment: unclear whether it was done correctly |
| Allocation concealment (selection bias) | Unclear risk | Comment: nothing reported, only description of syringes that were of same volume (2 mL plus 1 mL) for each intervention group. Unclear whether the allocation could have been foreseen |
| Blinding of participants and personnel (performance bias) Pain, 'rescue' analgesia, postoperative sedation | Unclear risk |
Quote: "this double‐blind trial..." Comment: insufficient information to allow a clear judgement |
| Blinding of participants and personnel (performance bias) PONV | Unclear risk |
Quote: "this double‐blind trial..." Comment: insufficient information to allow a clear judgement |
| Blinding of participants and personnel (performance bias) Time to first passage of flatus or stool | Unclear risk | Not relevant |
| Blinding of participants and personnel (performance bias) Time to first out‐of‐bed mobilization | Unclear risk | Not relevant |
| Blinding of participants and personnel (performance bias) Post‐interventional complications or side effects | Unclear risk |
Quote: "this double‐blind trial..." Comment: insufficient information to allow a clear judgement |
| Blinding of outcome assessment (detection bias) Pain, 'rescue' analgesia, postoperative sedation | Unclear risk |
Quote: "this double‐blind trial..." Comment: insufficient information to allow a clear judgement |
| Blinding of outcome assessment (detection bias) PONV | Unclear risk |
Quote: "this double‐blind trial..." Comment: insufficient information to allow a clear judgement |
| Blinding of outcome assessment (detection bias) Time to first passage of flatus or stool | Unclear risk | Not relevant |
| Blinding of outcome assessment (detection bias) Time to first out‐of‐bed mobilization | Unclear risk | Not relevant |
| Blinding of outcome assessment (detection bias) Post‐interventional complications or side effects | Unclear risk |
Quote: "this double‐blind trial..." Comment: insufficient information to allow a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data |
| Selective reporting (reporting bias) | High risk | No protocol available, and data concerning postoperative sedation are reported only as "no significance between groups" and no time points specified |
| Other bias | High risk | Nothing was reported about differences in anaesthetic agents between groups No reports on which type of surgery, other than major abdominal cancer surgery. Participants received an amount of 'rescue' analgesia during 24 hours postoperatively with a remarkably small standard deviation (see Analysis 1.2), which makes a more detailed report of anaesthetic agents and any difference between groups required |
Park 2012.
| Methods | RCT, 2 parallel groups | |
| Participants |
Number: 42 men (n = 19) and women (n = 23) Country: Korea Surgery: laparoscopic cholecystectomy, general anaesthesia, elective Age: 18 to 60 years ASA: I or II Other inclusion criteria: none reported Exclusion criteria: body mass index > 30 kg/m2, allergy to any medications, renal or hepatic insufficiency, neurological or psychiatric disease, preoperative heart rate < 45 beats/min, antihypertensive medication with clonidine or other alpha‐2 agonist |
|
| Interventions |
vs
All participants:
|
|
| Outcomes | VAS pain at 6 and 24 hours PO Total amount of intravenous 'rescue' tramadol at 24 hours PO (when the patient requested analgesics or had VAS pain > 4, 30 mg of ketorolac was injected intravenously; 30 minutes later if VAS was still higher than 4, patients received tramadol 50 mg intravenous boluses. If VAS pain was still greater than 4 after another 30 minutes, 20 µg of intravenous fentanyl was administered) |
|
| Notes | The amount of propofol differed between groups with (mg, mean, SD) 83 ± 23.4 in the dexmedetomidine group vs 117 ± 33.9 in the placebo group No report described the amount of rescue fentanyl. It was reported that 2 participants in the control group and none in the intervention group needed administration of fentanyl. No report on the number of participants needing ketorolac and tramadol; only total amounts administered were reported, showing a significant difference between groups for intravenous ketorolac (mg, mean, SD) with 43.5 ± 18 in the dexmedetomidine group vs 66 ± 39.6 in the placebo group Study authors contacted for details of relevance for risk of bias assessment, without luck |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "Patients were randomly allocated" Comment: Nothing else stated. Unclear how it was done |
| Allocation concealment (selection bias) | Unclear risk | Nothing reported |
| Blinding of participants and personnel (performance bias) Pain, 'rescue' analgesia, postoperative sedation | Unclear risk | Nothing reported |
| Blinding of participants and personnel (performance bias) PONV | Unclear risk | Not relevant |
| Blinding of participants and personnel (performance bias) Time to first passage of flatus or stool | Unclear risk | Not relevant |
| Blinding of participants and personnel (performance bias) Time to first out‐of‐bed mobilization | Unclear risk | Not relevant |
| Blinding of participants and personnel (performance bias) Post‐interventional complications or side effects | Unclear risk | Not relevant |
| Blinding of outcome assessment (detection bias) Pain, 'rescue' analgesia, postoperative sedation | Unclear risk |
Quote: VAS pain was assessed "after operation by an anaesthesiologist who was not involved in the study" Comment: Nothing stated about rescue analgesia, but one might assume that the analgesia was administered by the same person assessing VAS pain. But as nothing was reported about blinding of participants, risk of bias remained unclear |
| Blinding of outcome assessment (detection bias) PONV | Unclear risk | Not relevant |
| Blinding of outcome assessment (detection bias) Time to first passage of flatus or stool | Unclear risk | Not relevant |
| Blinding of outcome assessment (detection bias) Time to first out‐of‐bed mobilization | Unclear risk | Not relevant |
| Blinding of outcome assessment (detection bias) Post‐interventional complications or side effects | Unclear risk | Not relevant |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data |
| Selective reporting (reporting bias) | High risk | No protocol was available. Several outcomes were not prespecified but seemed to be determined post hoc, and use of antiemetics among patients with PONV was measured according to the Methods section but was not reported. Amount of rescue analgesia was not prespecified but probably was intended as an outcome pre hoc. Time points preselected for VAS pain were not exactly the same as those reported (prespecified time points 8 and 12 hours postoperatively were reported at 6 and 8 hours instead) |
| Other bias | Unclear risk | Insufficient information to allow a clear judgement |
Tufanogullari 2008.
| Methods | RCT, 4 parallel groups | |
| Participants |
Number: 80 men (n = 19) and women (n = 61) Country: USA Surgery: laparoscopic bariatric surgery (gastric banding or gastric bypass), general anaesthesia, elective Age: 22 to 66 years ASA: II or III Other inclusion criteria: none reported Exclusion criteria: allergy to alpha‐2 adrenergic agonists or sulpha drugs; uncontrolled hypertension; heart block greater than first degree; alcohol or drug abuse; neurological, cardiovascular, renal, hepatic or gastrointestinal disease; opioid analgesic medication within 24 hours; pregnancy or breast‐feeding; inability to speak and read English |
|
| Interventions |
vs
vs
vs
All participants:
|
|
| Outcomes | Amount of 'rescue' analgesia:
VRS pain 0 to 10 after 1 day PO Number of participants needing 'rescue' opioid after 1 day PO Number of participants with nausea during stay in PACU Number of participants with vomiting during stay in PACU Number of participants requiring antiemetics during stay in PACU (intravenous promethazine 6.25 mg administered if VRS nausea score > 3 on 2 consecutive evaluations) VRS nausea scores 0 to 10 at 30‐minute intervals until PACU discharge Time to passing flatus (participants making a diary note) Time to ambulation without assistance Number of participants with post‐interventional complications or side effects:
|
|
| Notes | Study authors contacted for supplementary information about 'rescue' analgesia regimen and details about PONV, without reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "...a computer‐generated random number table" Comment: probably done |
| Allocation concealment (selection bias) | Low risk |
Quote: "The study medication was prepared by the operating room (OR) pharmacist in identical 60‐mL syringes. DEX 0, 200, 400, or 800 µg was added to saline to achieve a total volume of 40 mL... for the 4 study groups...An infusion of the study medication was started at 0.04 mL/kg/h" Quote: "The investigators, attending anaesthesiologists, OR, recovery and ward nurses, as well as the patients were blinded to the computer‐generated randomization schedule" Comment: probably done |
| Blinding of participants and personnel (performance bias) Pain, 'rescue' analgesia, postoperative sedation | Low risk |
Quote: "The investigators, attending anaesthesiologists, OR, recovery and ward nurses, as well as the patients were blinded to the computer‐generated randomization schedule" Comment: probably done |
| Blinding of participants and personnel (performance bias) PONV | Low risk | Same as above |
| Blinding of participants and personnel (performance bias) Time to first passage of flatus or stool | Low risk | Same as above |
| Blinding of participants and personnel (performance bias) Time to first out‐of‐bed mobilization | Low risk | Same as above |
| Blinding of participants and personnel (performance bias) Post‐interventional complications or side effects | Low risk | Same as above |
| Blinding of outcome assessment (detection bias) Pain, 'rescue' analgesia, postoperative sedation | Low risk | Same as above |
| Blinding of outcome assessment (detection bias) PONV | Low risk | Same as above |
| Blinding of outcome assessment (detection bias) Time to first passage of flatus or stool | Low risk | Same as above |
| Blinding of outcome assessment (detection bias) Time to first out‐of‐bed mobilization | Low risk | Same as above |
| Blinding of outcome assessment (detection bias) Post‐interventional complications or side effects | Low risk | Same as above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Quote: "Three patients (one from each of the DEX 0.2, 0.4, and 0.8 groups) were admitted to the intensive care unit from the postsurgical ward because of surgical complications at the gastrointestinal anastomosis site (e.g., bleeding, obstruction) and their postoperative data were excluded from the final analysis" Comment: As participants were excluded for surgical reasons and were equally distributed, risk of bias was probably low |
| Selective reporting (reporting bias) | Unclear risk | The published article was in line with the protocol, which was available, but outcomes were described in very general terms only. Two outcomes seemed to be added post hoc (number of participants discharged on postoperative day 1 and number of days until discharge), but they showed no significant differences between groups |
| Other bias | High risk |
Comment: Criteria for the use of analgesia were not clearly described, even though participants went through a complex 3‐step rescue analgesia regimen. As VRS pain scores were collected only in the PACU and on postoperative days 1 and 2, one might suspect some irregularities in the way analgesia was administered. Information given to participants on how to administer the analgesia at home was not described. One might suspect lack of control of participant compliance at home, leading to high risk of bias Quote: "This investigator–initiated, Food and Drug Administration‐approved study was supported, in part, by an unrestricted educational grant from Hospira, Inc.a (Lake Forest, IL), endowment funds from the Margaret Milam McDermott Distinguished Chair in Anesthesiology, and the White Mountain Institute, a non‐profit private foundation (Paul F. White, President)" Quote (from authors' conclusion): "Our findings would suggest that the modest anaesthetic‐sparing effect was of little (if any) clinical significance because dexmedetomidine failed to facilitate a faster emergence from desflurane anaesthesia after bariatric surgery. (…) the primary benefit of Dex in this study appeared to be related to its ability to reduce emetic sequelae by decreasing the need for the desflurane during the operation and fentanyl immediately after surgery" Comment: The potential conflict of interest is of unclear importance. The conclusion of the study authors seem modest and reasonable, but it is not stated in what way the grant was 'unrestricted' (e.g. if Hospira owned the data or needed to approve of the manuscript) |
Xiao 2013.
| Methods | RCT, 3 parallel groups | |
| Participants |
Number: 120 men (n = 53) and women (n = 67) Country: China Surgery: open abdominal surgery, general anaesthesia, elective Age: 18 to 60 years ASA: I or II Other inclusion criteria: expected duration of surgery > 45 minutes Exclusion criteria: body mass index > 28 kg/m2; metabolic diseases such as diabetes, hyperthyroidism; serious cardiovascular disease; alcohol or illicit drugs; mental illness or epilepsy |
|
| Interventions |
vs
vs
All participants:
|
|
| Outcomes | Dose of 'rescue' morphine at 12 and 24 hours PO Postoperative pain measured by VAS at 12 and 24 hours Postoperative sedation measured by RSS at 12 and 24 hours |
|
| Notes | This review looked at only 2 of the 3 parallel groups in this study Amounts of intraoperative sevoflurane, remifentanil and phenylephrine did not differ significantly between the 2 groups analysed by this review. Investigators reported significant differences for these 3 drugs when compared with low‐dose remifentanil Study available only in Chinese. Qualitative data extracted by only 1 person, the translator, whereas quantitative data were extracted by the review authors from tables with no need of translation. Study authors contacted in English for additional information of relevance for risk of bias assessment, type of surgery, criteria for 'rescue' analgesia, etc, without luck |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "...random number table was used to generate random sequence..." Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | Nothing reported |
| Blinding of participants and personnel (performance bias) Pain, 'rescue' analgesia, postoperative sedation | Unclear risk |
Quote: "this is a double‐blind randomized controlled trial” Comment: nothing else reported, unclear how blinding was done |
| Blinding of participants and personnel (performance bias) PONV | Unclear risk | Not relevant |
| Blinding of participants and personnel (performance bias) Time to first passage of flatus or stool | Unclear risk | Not relevant |
| Blinding of participants and personnel (performance bias) Time to first out‐of‐bed mobilization | Unclear risk | Not relevant |
| Blinding of participants and personnel (performance bias) Post‐interventional complications or side effects | Unclear risk |
Quote: "this is a double‐blind randomized controlled trial” Comment: nothing else reported, unclear how blinding was done |
| Blinding of outcome assessment (detection bias) Pain, 'rescue' analgesia, postoperative sedation | Unclear risk | Same as above |
| Blinding of outcome assessment (detection bias) PONV | Unclear risk | Not relevant |
| Blinding of outcome assessment (detection bias) Time to first passage of flatus or stool | Unclear risk | Not relevant |
| Blinding of outcome assessment (detection bias) Time to first out‐of‐bed mobilization | Unclear risk | Not relevant |
| Blinding of outcome assessment (detection bias) Post‐interventional complications or side effects | Unclear risk |
Quote: "this is a double‐blind randomized controlled trial” Comment: nothing else reported, unclear how blinding was done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data |
| Selective reporting (reporting bias) | Unclear risk | All outcomes described in the Methods section were accounted for in the Results section |
| Other bias | Unclear risk | Incomplete information to allow a clear judgement |
RCT: randomized controlled trial; ASA: American Society of Anesthesiologists Physical Status 1 to 6, where 1 = Healthy person, 2 = Mild systemic disease, 3 = Severe systemic disease, 4 = Severe systemic disease that is a constant threat to life, 5 = A moribund person who is not expected to survive without the operation, 6 = A declared brain‐dead person whose organs are being removed for donor purposes; BIS: bispectral index, a measure of electroencephalography (EEG)‐based depth of anaesthesia; PCA: patient‐controlled analgesia; VAS: visual analogue scale; PO: postoperative; SD: standard deviation; PONV: postoperative nausea and vomiting; PACU: post‐anaesthesia care unit; DEX: dexmedetomidine; RSS: Ramsey Sedation Scale 1 to 6, where 1 = Patient is anxious and agitated or restless or both; 2 = Patient is co‐operative, oriented and tranquil, 3 = Patient responds to commands only, 4 = Patient exhibits brisk response to light glabellar tap or loud auditory stimulus, 5 = Patient exhibits a sluggish response to light glabellar tap or loud auditory stimulus, 6 = Patient exhibits no response. aHospira is a manufacturer of dexmedetomidine
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abdalla 2003 | Cystectomy |
| Akinci 2011 | Three intervention groups with different doses of dexmedetomidine, but no control group |
| Aldehayat 2011 | No relevant outcomes |
| Arain 2002 | Hernia repair and orthopaedic surgery |
| Basar 2008 | No relevant outcomes |
| Bergese 2010 | No relevant outcomes |
| Bhattacharjee 2010 | No relevant outcomes |
| Candiotti 2010 | Not abdominal surgery |
| Chen 2013 | No relevant outcomes |
| Elcicek 2010 | Lower extremity surgery |
| Gupta 2011 | Gynaecological and urological surgery and hernia repair |
| Gupta 2014a | Abdominal hysterectomy and inguinal hernioplasty |
| Gupta 2014b | Plastic and otorhinolaryngological surgery, but also regional 'general surgery' of duration about 1 hour. Study authors emailed to confirm which type, but no reply. Excluded as it probably was not abdominal surgery |
| Gurbet 2006 | Intra‐abdominal hysterectomy |
| Guryanov 2013 | Intensive care unit (in Russian only, data extracted on a voluntary basis by a native Russian medical search expert within The Cochrane Collaboration) |
| Harsoor 2014 | Relevant outcomes are not reported at time points collected by this review. Ramsay Sedation Scale and visual analogue scale (VAS) pain are reported only after 1 hour postoperatively |
| Iwakiri 2012 | Gynaecological surgery |
| Jakob 2012 | Dexmedetomidine given for sedation in intensive care unit |
| Kaya 2010 | Urological surgery |
| Keniya 2011 | Not abdominal surgery |
| Lawrence 1997 | Only orthopaedic and superficial surgery (hernia repair, pilonidal sinus, varicose veins, hydrocoele) |
| Marangoni 2005 | Dexmedetomidine not the sole difference between intervention and control groups. The study compared sufentanil vs dexmedetomidine plus midazolam |
| Málek 2010 | Intervention groups with too many agents and interactions:
vs
vs
vs
|
| No author 2007 | Abdominal hysterectomy |
| Ohtani 2008 | Gynaecological surgery |
| Ozbakis 2008 | Gynaecological and urological surgery (full text not retrievable, but information about specific type of surgery was found in Schnabel 2013) |
| Padma 2013 | Dexmedetomidine not administered perioperatively |
| Shukla 2011 | No relevant outcomes |
| Tasdogan 2009 | Dexmedetomidine administered in intensive care unit and not perioperatively |
| Wan 2011 | Dexmedetomidine administered in intensive care unit and not perioperatively |
| Yildiz 2006 | Other minor surgery |
| Zeng 2014 | Nephrectomy |
Characteristics of studies awaiting assessment [ordered by study ID]
Altindis 2008.
| Methods | RCT, 2 parallel groups |
| Participants |
Number: 40 participants Country: Turkey Surgery: lower abdominal surgery Age: 21 to 56 years ASA: I or II Other inclusion criteria: general anaesthesia Exclusion criteria: history of hypertension, ischaemic heart disease or conduction disturbance; history of alcohol or drug abuse; use of beta‐adrenoreceptor blockers, monoamine oxidase inhibitor within 2 weeks; impaired hepatic or renal function; hypersensitivity to opioids or dexmedetomidine |
| Interventions |
vs
All participants:
|
| Outcomes | Verbal rating score for pain (0 to 3) Total meperidine consumption Time to discharge from PACU Postoperative sedation (0 to 3) Number of participants with postoperative vomiting Hemodynamic data |
| Notes | Study authors contacted to clarify types of surgery. No reply |
Anvaroglu 2008.
| Methods | RCT, 2 parallel groups |
| Participants |
Number: 50 participants, all female Country: Turkey Surgery: abdominal surgery Age: 20 to 60 years ASA: I to II Other inclusion criteria: general anaesthesia Exclusion criteria: none stated |
| Interventions |
vs
|
| Outcomes | Total analgesic consumption |
| Notes | Type of surgery unknown. We were unable to locate anything but an abstract. Study authors would have been contacted if we could have located an address |
Arain 2004.
| Methods | RCT, 2 parallel groups |
| Participants |
Number: 34 participants (gender not reported) Country: USA Type of surgery: intra‐abdominal (15), major orthopaedic (13), other (6) Age: adults, mean age 60 years ASA: I to III Other inclusion criteria: normal renal function, scheduled for at least a 24‐hour stay in the hospital Exclusion criteria: second‐ or third‐degree heart block, use of any experimental drug, including dexmedetomidine or other alpha‐2 agonists within 28 days, long‐term use of medical therapy that might influence the outcome of the study (such as opioids), current history of psychiatric disorder or presently taking psychotropic medications, ejection fraction < 30%, sleep apnoea, BMI > 35 kg/m2 |
| Interventions |
vs
All participants:
|
| Outcomes | VAS postoperative sedation (0 to 100 mm, where 100 = wide awake) regularly during 100 minutes after surgery VAS postoperative pain regularly during first 100 minutes after surgery VAS 0 to 100 postoperative nausea during first 100 minutes after surgery Number of participants with need for additional morphine at 30 and 60 minutes after surgery Amount of 'rescue' morphine during 30 minutes after surgery Amount (increments) of PCA morphine during 24 hours on the ward (no total, but frequent time points) |
| Notes | PCA morphine data were lost from 4 participants (2 from each group) Study authors contacted for individual participant data, as this review does not look at orthopaedic surgery. No reply |
Bicer 2006.
| Methods | RCT, 3 parallel groups |
| Participants |
Number: 120 men (n = 74) and women (n = 46) Country: Turkey Surgery: elective abdominal and orthopaedic, lasting 1 to 3 hours Age: 18 to 50 years ASA: I to II Other inclusion criteria: none stated Exclusion criteria: BMI > 27 kg/m2; fever; use of vasoactive, antidepressant or analgesic drugs; history of cardiovascular, respiratory, endocrine or neurological disease; pregnancy |
| Interventions |
vs
vs
|
| Outcomes | Postoperative sedation, grade 1 to 6 Postoperative pain, grade 0 to 4 Postoperative nausea and vomiting Postoperative analgesic requirements (not specifying type of analgesia) Postoperative antiemetic requirements |
| Notes | Study authors contacted for type of abdominal surgery and individual participant data, and to obtain data on type of rescue analgesia and data on postanaesthetic pain. Study authors sent 1 reply, but not to the above mentioned details. Awaiting new reply |
Ceballos 2011.
| Methods | RCT, 2 parallel groups |
| Participants |
Number: 91 men (n = 46) and women (n = 45) Country: Mexico Surgery: bariatric, no description of open/laparoscopic (probably laparoscopic because they stated that modern technique was used) Age: 18 to 65 years ASA: not reported (probably primarily ASA II) Other inclusion criteria: BMI > 32 kg/m2, less than 10 years of diabetes, less than 5 years of treatment with insulin, proper control of co‐morbidity Exclusion criteria: none reported |
| Interventions |
vs
All participants:
|
| Outcomes | VAS pain 0 to 10 at 1 and 12 hours after surgery (converted by study authors post hoc to a dichotomous value: VAS 0 to 5 = no pain, and VAS 5 to 10 = pain) Ramsay Sedation Scale at emersion and at 1 hour after surgery |
| Notes | Baseline imbalance regarding gender: men/women in dexmedetomidine group vs fentanyl group, respectively, 32/13 and 14/32 Need for fentanyl was different between groups: dexmedetomidine group required a mean (± SD) of 3.7 (± 0.99) μg/kg/h, whereas fentanyl group required 5.5 (± 0.68) μg/kg/h Study authors contacted for details on amount and type of rescue analgesia, and for not‐dichotomized VAS pain scores, without reply |
Kilicaslan 2006.
| Methods | RCT, 2 parallel groups |
| Participants |
Number: 25 participants Country: Turkey Surgery: elective lower abdominal surgery (including gynaecology) Age: adults ASA: I to II Other inclusion criteria: not stated Exclusion criteria: not stated |
| Interventions |
vs
|
| Outcomes | Haemodynamics preoperatively, perioperatively and postoperatively Desflurane requirements Catecholamine levels Sedation, pain and recovery scores postoperatively |
| Notes | Study authors contacted for individual information on participants undergoing abdominal and not gynaecological operations, but no reply |
Kordan 2006.
| Methods | RCT, 3 parallel groups |
| Participants |
Number: 45 participants Country: Turkey Surgery: not stated Age: not stated ASA: not stated Other inclusion criteria: not stated Exclusion criteria: not stated |
| Interventions |
vs
vs
|
| Outcomes | Haemodynamics perioperatively and postoperatively Anaesthetic requirements Sedation scores postoperatively Recovery score Side effects |
| Notes | We were unable to locate anything but an abstract. Study authors would have been contacted to specify outcomes and type of surgery if we could have found an address |
Mizrak 2010.
| Methods | RCT, 2 parallel groups |
| Participants |
Number: 90 participants Country: Turkey Surgery: 24 inguinal hernia, 32 laparoscopic cholecystectomy, 34 breast biopsy under general anaesthesia Age: 18 to 60 years ASA: I to II Inclusion criteria: none stated Exclusion criteria: heart blocks, heart failure, hepatic failure, psychiatric disease, neurological disease, drug allergy, analgesics or sedatives within previous 24 hours |
| Interventions |
vs
vs
|
| Outcomes | Recovery time VAS postoperative pain Side effects including headache, nausea, vomiting, coughing and fever |
| Notes | Study authors contacted for individual participant data for abdominal surgery. No reply |
Scheinin 1992.
| Methods | RCT, 2 parallel groups |
| Participants |
Number: 24 men (n = 11) and women (n = 13) Country: Finland Surgery: laparotomy, breast surgery, anal surgery, various surgery Age: adults ASA: I Other inclusion criteria: none stated Exclusion criteria: use of any medication, childbearing potential, known allergy |
| Interventions |
vs
|
| Outcomes | Analgesic requirements during the first 2 hours postoperatively |
| Notes | Study authors contacted to obtain specific data for abdominal surgery, but no reply (study from 1992) |
Subasi 2012.
| Methods | RCT, 2 parallel groups |
| Participants |
Number: 40 participants Country: Turkey Surgery: laparoscopic cholecystectomy Age: 18 to 60 years ASA: I to II Other inclusion criteria: none stated Exclusion criteria: not stated |
| Interventions |
vs
All participants:
|
| Outcomes | Time to recovery Postoperative analgesic requirements Postoperative haemodynamic parameters Postoperative pain scores Time to first analgesic need |
| Notes | Only conference abstract available. Study authors contacted to obtain more information, but no reply |
Unlugenc 2005.
| Methods | RCT, 2 parallel groups |
| Participants |
Number: 60 men (n = 21) and women (n = 39) Country: Turkey Surgery: mini‐laparotomy (3), cholecystectomy (30), splenectomy (4), inguinal, incisional or umbilical hernia repair (23) Age: 18 to 64 years ASA: I to II Other inclusion criteria: none reported Exclusion criteria: inability to use the PCA device, long‐term use of opioids and history of chronic pain |
| Interventions |
vs
All participants:
|
| Outcomes | The following outcomes measured at 2, 6, 12 and 24 hours after start of PCA morphine:
|
| Notes | Study authors contacted for individual participant data, because this review does not include hernia repair. No reply |
Yacout 2012.
| Methods | RCT, 2 parallel groups |
| Participants |
Number: 30 men (n = 17) and women (n = 13) Country: Egypt Surgery: major abdominal surgery (not specified which type or if laparoscopic/open), elective, general anaesthesia Age: range not reported, mean age in dexmedetomidine group 49.6 and in placebo group 47.1 years ASA: I to III Other inclusion criteria: none mentioned Exclusion criteria: none mentioned |
| Interventions |
vs
All participants:
|
| Outcomes | VAS pain at 6, 12 and 24 hours postoperatively Amount of ketorolac during 24 hours postoperatively |
| Notes | This study reported no standard deviations or other variation for results, but found a 'significant difference' with a mean dose of ketorolac 24 hours after surgery at 3.8 mg in the dexmedetomidine group vs 16.0 mg in the placebo group. No opioids were used. VAS postoperative pain was with 'no significant difference' between groups at 6, 12 and 24 hours after surgery (only during the first postoperative hour, a significant difference in favour of dexmedetomidine was found). The study reported no side effects. We tried to contact study authors for information about standard deviations, type of abdominal surgery, etc, but with no response |
Yektas 2011.
| Methods | RCT, 9 parallel groups |
| Participants |
Number: 180 participants (9 × 20) Country: Turkey Surgery: inguinal surgery Age: 20 to 30 years ASA: I Other inclusion criteria: spinal anaesthesia Exclusion criteria: drug abuse, recall of pain at earlier surgery, need for additional sedation at earlier surgery, cerebrospinal fluid could not be obtained after 3 attempts, education level below primary school |
| Interventions | Control group: 15 mg hyperbaric bupivacaine and 0.5 mL physiological serum intrathecally vs In the other groups, serum was replaced with the following to a total volume of 3.5 mL:
vs
vs
vs
vs
vs
vs
vs
|
| Outcomes | Intraoperative and postoperative side effects Time to first pain |
| Notes | Only abstract of conference paper available. Study authors contacted for additional information, awaiting reply |
RCT: randomized controlled trial; ASA: American Society of Anesthesiologists Physical Status 1 to 6, where 1 = Healthy person, 2 = Mild systemic disease, 3 = Severe systemic disease, 4 = Severe systemic disease that is a constant threat to life, 5 = A moribund person who is not expected to survive without the operation, 6 = A declared brain‐dead person whose organs are being removed for donor purposes; PCA: patient‐controlled analgesia; PACU: post‐anaesthesia care unit; BMI: body mass index
Characteristics of ongoing studies [ordered by study ID]
Awad 2014.
| Trial name or title | Intravenous Dexmedetomidine for the Prevention of Postoperative Shivering in Patients Undergoing General Anaesthesia |
| Methods | RCT, 4 parallel groups |
| Participants |
Number: 216 participants, both genders Country: Lebanon Surgery: elective surgery under general anaesthesia with estimated time of 1 to 3 hours ‐ type not stated Age: 18 to 80 years ASA: I to III Other inclusion criteria: none stated Exclusion criteria: duration of surgery < 1 hour or > 3 hours; allergy to dexmedetomidine, vasoactive antidepressant or analgesics; BMI > 30 kg/m2, fever, pregnancy |
| Interventions |
vs
vs
vs
|
| Outcomes | Time to extubation, awakening and orientation Sedation scores up to 1 hour postoperatively Pain scores up to 1 hour postoperatively Nausea and vomiting up to 1 hour postoperatively |
| Starting date | May 2014 |
| Contact information | Dr. Marie Awad, Professor of Clinical Specialty, American University of Beirut Medical Center mm01@aub.edu.lb |
| Notes | Study authors contacted to obtain data on type of surgery, but no reply Status at clinicaltrials.gov: recruiting, last verified May 2014 Clinical Trials identifier: NCT02141412 |
Jung 2014.
| Trial name or title | Effect of Dexmedetomidine on Recovery Profiles of Elderly Patients |
| Methods | RCT, 4 parallel groups |
| Participants |
Number: 120 participants, both genders Country: Korea Surgery: not stated, elective Age: > 65 years ASA: I to II Other inclusion criteria: not stated Exclusion criteria: severe heart disease (New York Heart Association class > III, severe arrhythmia, uncontrolled hypertension or hypotension, hypersensitivity to drugs, cognitive deficiency, dementia or delirium, hepatic or renal impairment, infective disease |
| Interventions |
vs
vs
vs
|
| Outcomes | Recovery characteristics (time to recovery of consciousness (ROC) and recovery) Bispectral index (BIS) values at ROC and orientation Ricker sedation‐agitated scale at the postanaesthetic care unit Safety (vital signs during and after administration of dexmedetomidine) |
| Starting date | May 2013 |
| Contact information | Study Chair: Ki Tae Jung, MD, Department of Anesthesiology and Pain Medicine, School of Medicine, Chosun University, Gwangju, Korea, 501‐717 |
| Notes | Status at clinicaltrials.gov: completed, last verified March 2014, no results posted Clinical Trials identifier: NCT01851005 Type of surgery not stated ‐ might not be abdominal surgery |
Kim 2014.
| Trial name or title | The Effect of Dexmedetomidine on Postoperative Analgesia |
| Methods | RCT, 2 parallel groups |
| Participants |
Number: 95 participants, both genders Country: Korea Surgery: elective surgery for colon cancer Age: 20 to 74 years ASA: not stated Other inclusion criteria: none stated Exclusion criteria: hepatic or renal disease, allergy to study drugs, inability to use patient‐controlled analgesia |
| Interventions |
vs
|
| Outcomes | Total amount of administered fentanyl during first 24 hours after surgery |
| Starting date | June 2011 |
| Contact information | Study Chair: Yong Chul Kim, Professor Seoul National University Hospital, Seoul, Korea, 110‐744 |
| Notes | Details of drug administration or timing not available Status at clinicaltrials.gov: completed, last verified March 2012, no results posted Clinical Trials identifier: NCT01373021 |
Wai 2014.
| Trial name or title | Multimodal Analgesic Using Morphine and COX‐2 With or Without Dexmedetomidine for Colorectal Surgery |
| Methods | RCT, 2 parallel groups |
| Participants |
Number: 100 participants, both genders Country: Hong Kong Surgery: colorectal Age: 18 to 80 years ASA: I to III Other inclusion criteria: none stated Exclusion criteria: extended resection involving other organs such as liver and urinary bladder; allergy to alpha‐2 agonists, opioids, non‐steroidal anti‐inflammatory drugs (NSAIDs) including COX‐2 inhibitors or sulphonamides; regular use of clonidine, methyldopa, opioids or psychiatric drugs; alcohol or drug abuse; second‐ or third‐degree heart block; ischaemic heart disease, valvular heart disease or heart failure; history of pulmonary embolism or deep vein thrombosis; sleep apnoea; impaired renal function, defined as preoperative serum creatinine level over 120 µmol/L; impaired hepatic function, defined as preoperative serum albumin level < 30 g/L; impaired or retarded mental state; not self ambulatory before operation; difficulties in using patient‐controlled analgesia; BMI > 35 kg/m2; pregnancy; patient refusal |
| Interventions |
vs
|
| Outcomes | Postoperative pain score on numerical rating scale during 5 days Number of participants with flatus during 5 days |
| Starting date | May 2008 |
| Contact information | Cheung Chi Wai, Clinical Associate Professor, The University of Hong Kong; nothing else stated |
| Notes | Status at clinicaltrials.gov: completed, last verified April 2013, no results posted Clinical Trials identifier: NCT01353456 |
Yoo 2014.
| Trial name or title | The Effects of Intraoperative Dexmedetomidine Infusion on Postoperative Bowel Movement in Patients Undergoing Laparoscopic Gastrectomy |
| Methods | RCT, 2 parallel groups |
| Participants |
Number: 92 participants, both genders Country: Korea Surgery: elective laparoscopic gastrectomy Age: 20 to 65 years ASA: I to III Other inclusion criteria: gastric cancer Exclusion criteria: ASA physical status Ⅳ, bradycardia (< 60 beats/min), arrhythmia, uncompensated heart failure, hepatic failure, renal failure |
| Interventions |
vs
|
| Outcomes | Time to first gas passing Time to intake of sips of water Time to intake of soft diet |
| Starting date | June 2014 |
| Contact information | Young Chul Yoo, MD, Department of Anesthesiology and Pain Medicine, Yonsei University College of Medicine, Seoul, Korea, Republic of, 120‐752, seaoyster@yuhs.ac |
| Notes | Status at clinicaltrials.gov: recruiting patients, last verified June 2014 Clinical Trials identifier: NCT02164448 |
Zeeni 2014.
| Trial name or title | Dexmedetomidine for Postoperative Analgesia After Bariatric Surgery |
| Methods | RCT, 2 parallel groups |
| Participants |
Number: 60 participants, both genders Country: Lebanon Surgery: laparoscopic sleeve gastrectomy bariatric surgery Age: 18 to 70 years ASA: I to II Other inclusion criteria: body mass index > 40 kg/m2 or > 35 kg/m2 with co‐morbidity such as hypertension, diabetes or sleep apnoea Exclusion criteria: allergy to opioids or alpha‐2 agonists; weight > 180 kg; uncontrolled hypertension; heart block greater than first degree; prolonged QT interval; clinically significant neurological, cardiovascular, renal, hepatic or gastrointestinal disease; opioid analgesic medication within 24 hours; history of alcohol, drug abuse or long‐term opioid intake or psychiatric disorder; pregnancy or breast‐feeding |
| Interventions |
vs
|
| Outcomes | Total dose of morphine consumed in PACU Time to first morphine requirement in PACU Numerical rating scale (NRS) for pain NRS for nausea Incidence of pruritus Incidence of vomiting or retching Incidence of respiratory complications Time to discharge readiness in PACU Total morphine consumption at 24 hours Quality of recovery (QoR‐40) score at 24 hours Overall satisfaction at 1 month |
| Starting date | August 2014 |
| Contact information | American University of Beirut Medical Center, Beirut, Lebanon Contact: Carine Zeeni, MD, 961 1 350000 ext 6380, cz07@aub.edu.lb Contact: Sahar Siddik, MD, 961 1 350000 ext 6380, ss01@aub.edu.lb |
| Notes | Status at clinicaltrials.gov: recruiting patients, last verified July 2014 Clinical Trials identifier: NCT02213159 |
RCT: randomized controlled trial; ASA: American Society of Anesthesiologists Physical Status 1 to 6, where 1 = Healthy person, 2 = Mild systemic disease, 3 = Severe systemic disease, 4 = Severe systemic disease that is a constant threat to life, 5 = A moribund person who is not expected to survive without the operation, 6 = A declared brain‐dead person whose organs are being removed for donor purposes; BMI: body mass index
Differences between protocol and review
Review information
Second author Helene Korvenius Nedergaard changed her surname from Jørgensen after publication of the protocol (Jessen 2013).
Background
The Background section has been updated with references until 2014.
We have clarified that the same drug dexmedetomidine is sold as Precedex and Dexdor under two different regulatory authorities.
In our protocol, we stated both Dexdor product information and Precedex prescribing information as references for the following sentence: "Infusion (of dexmedetomidine) exceeding 24 hours is not recommended due to the risk of agitation and respiratory failure". This was not correct and has now been amended to read as follows: "Infusion of dexmedetomidine exceeding 24 hours is not recommended because of risk of agitation and respiratory failure (Precedex prescribing information); this precaution is not stated in Dexdor product information".
To adverse events, we have added sinus arrest, as described by Dexdor product information and Precedex prescribing information.
Types of participants
After our protocol (Jessen 2013) had been published, we narrowed our review question regarding type of surgery, from abdominal procedures including gynaecological surgery, to only abdominal surgery. We made this decision to diminish heterogeneity in outcomes such as postoperative pain, gastrointestinal function and mobilization, which we visualized would be difficult to pool if surgery varied from peritoneal to vaginal procedures. The decision was made at a point when more than 30 studies seemed to meet inclusion criteria, but before full text of studies was read and before attention was paid to study results. Therefore, the decision probably introduced no risk of bias, although the reasons for making this decision can be discussed. We have added the following definition of abdominal surgery to the review: "Abdominal surgery was defined as surgery to intra‐abdominal organs, excluding gynaecological, urological, vascular and superficial surgery (such as hernia repair)".
Types of outcome measures
In our primary outcome, we have changed 'dose' to 'amount'.
Search methods for identification of studies
The following sentence was moved from the section Searching other resources to the section Electronic searches: "We applied no language restrictions".
Data extraction and management
We decided post hoc to supplement the collection of baseline data with height and weight of participants. The fact that several studies looked at bariatric surgery influenced this decision, but because this represents baseline information and not an outcome, it probably did not introduce risk of bias.
Assessment of heterogeneity
In the protocol, we stated: "A meta‐analysis would only be appropriate ... if variation in results was not considerable (I2 statistic < 75%) and if the amount of information was sufficient (size and number of trials)".
In the review, we added the following clarification: "A meta‐analysis would be appropriate ... only if variation in results was not considerable, as judged by clinical and methodological measures and by the statistical measure of heterogeneity, the I2 statistic, which ideally but not necessarily should be below 75% (Higgins 2011). Additionally, a meta‐analysis would be appropriate only if the amount of information was sufficient (size and number of trials) (Higgins 2011)".
Assessment of risk of bias in included studies
The following paragraph has been removed: “We will judge a study as having a low risk of bias if there is low risk of bias in all the domains. However, we will consider the likely direction and magnitude of bias. We will also take into consideration the relative importance of different domains according to the outcome in question. We will consider a study as having a high risk of bias if there is one or several domains with a high risk of bias. We will consider a study as having an unclear risk of bias, if there is insufficient information in one or several domains to assess the risk of bias”. This paragraph was removed because we found it more accurate to assess only risk of bias of outcomes and domains, not of a whole study.
We grouped outcomes in our risk of bias table post hoc, but before we looked into results of studies; therefore this probably did not introduce any risk of bias.
Unit of analysis issues
We removed the following sentences from the section Unit of analysis issues in the protocol: "For any non‐standard design, we will ‐ in our analysis ‐ account for the level at which randomization occurred....For any cluster‐randomized trial, we will choose one of two methods of analysis. Depending on the number and size of the clusters, we will decide whether to perform the analysis at the same level as the allocation (i.e. where the sample size is the number of clusters), or to perform the analysis at the level of the individual while using a statistical method taking into account the clustering in the data". These methodological considerations turned out to be irrelevant because no studies applied to the problem described. These considerations will be applied in future updates of this review, if relevant.
Dealing with missing data
Since our protocol was published (Jessen 2013), we have added information on how we contacted study authors: "For any type of missing data, we contacted the first author of the relevant trial to ask for additional information. If the contact information was not directly available, we tried to retrieve an email address or a postal address by searching the Internet, or by contacting co‐authors from present or former studies. Some attempted correspondence may not have reached the addressee".
Assessment of reporting biases
The section Assessment of reporting biases has been simplified as we were not able to create a funnel plot because the number of included studies was too small: "If the studies are not too similar in sizes (and standard errors), we intend to conduct a test for funnel plot asymmetry. The result will be correlated with visual interpretation of the funnel plot. For continuous outcomes with intervention effects measured as mean differences, we intend to use the test proposed by Egger et al (Egger 1997) for funnel plot asymmetry. The test consists in a linear regression of intervention effect estimate against its standard error, weighted by the inverse of the variance of the intervention effect estimate. For all the other of our preferred outcome measures, a test for funnel plot asymmetry is not recommended (Higgins 2011). Hence, any asymmetry will be ascribed by visual interpretation alone. In the case of funnel plot asymmetry, publication bias is only one of several possible explanations. Poor methodological quality in smaller studies is another common explanation. We will seek to understand the most probable source of the asymmetry and perform sensitivity analyses accordingly". If future updates of this review make funnel plots possible, we plan to do as described.
Data synthesis
The section Data synthesis has been shortened as we did not perform meta‐analyses because data were few and heterogeneity across studies was considerable. In the protocol, we stated: "As mentioned in the Assessment of heterogeneity section, we plan to perform a meta‐analysis if heterogeneity is not considerable. At the same time, we expect a certain amount of variation between studies. The effect of dexmedetomidine on the outcomes selected is likely to vary with different types of participants (types of surgery, types of comorbidity), and with different types of intervention (variation in administration, dosage and duration). Consequently, we plan to perform a random‐effects meta‐analysis. For all selected outcomes (except post‐interventional complications or adverse effects), we intend to use a variant of the inverse variance meta‐analysis method, the DerSimonian and Laird method (DerSimonian 1986), in which the standard errors are adjusted to the estimated variation among intervention effects. As a sensitivity analysis, we intend to perform a fixed‐effect meta‐analysis. In this context, we will choose the Mantel‐Haenszel method (Greenland 1985; Mantel 1959) for dichotomous outcomes when number of participants or studies is small. Among our selected outcomes, we do not expect post‐interventional complications and adverse effects to be suitable for a meta‐analysis or for the Summary of findings table. Instead, we intend to make a narrative report". If future updates of this review make meta‐analyses appropriate, we plan to do as described.
Subgroup analysis and investigation of heterogeneity
In the section Subgroup analysis and investigation of heterogeneity, we stated the following in the protocol (Jessen 2013).
"In order to investigate heterogeneity, we plan to undertake the following subgroup analyses, if required:
laparoscopic procedures versus open procedures;
gynaecological surgery versus other types of abdominal surgery;
single‐dose versus infusion (with or without loading dose); and
route of administration".
If future updates of this review make subgroup analyses possible, we plan to do as described. However, we will not perform the subgroup analysis of gynaecological surgery versus other types of abdominal surgery for the reasons stated under Types of participants in this section.
Sensitivity analysis
In the section Sensitivity analysis, we stated the following in the protocol.
"In order to clarify if any findings of our meta‐analyses are robust to the arbitrary decisions made during the review process, we intend to perform several sensitivity analyses as appropriate given the data available:
exclusion of studies at high risk of bias, as well as exclusion of studies at high and unclear risk of bias;
change in measures of intervention effect (e.g. from RR to odds ratio, from MD to SMD, if possible), in the case of substantial heterogeneity;
comparison of random‐effects with fixed‐effect meta‐analysis. This sensitivity analysis will help to clarify the reasons for any asymmetry in funnel plots. If the random‐effects model results in more beneficial intervention effects, it will indicate small‐study effects (arising from publication bias or from poor methodological quality or both) and not just artefact, true heterogeneity or chance as the cause of asymmetry. In this case, we will consider excluding smaller studies in another sensitivity analysis; and
trim‐and‐fill method to identify and correct publication bias expressed as funnel plot asymmetry".
If future updates of this review make sensitivity analyses possible, we plan to do as described.
Summary of findings
As no meta‐analyses were performed, we did not make use of the GRADE software available for assessing quality of evidence, as specified in the protocol.
Contributions of authors
Luise Jessen Lundorf (LJL), Helene Korvenius Nedergaard (HKN), Ann Merete Møller (AMM).
Conceiving of the review: LJL, AMM.
Co‐ordinating the review: LJL.
Undertaking manual searches: LJL.
Screening search results: LJL.
Organizing retrieval of papers: LJL.
Screening retrieved papers against inclusion criteria: LJL, HKN.
Appraising quality of papers: LJL, HKN, AMM.
Abstracting data from papers: LJL, HKN.
Writing to authors of papers for additional information: LJL.
Providing additional data about papers: LJL, HKN.
Obtaining and screening data on unpublished studies: LJL.
Managing data for the review: LJL, HKN.
Entering data into Review Manager (RevMan 5.3): LJL, HKN.
Analysing RevMan statistical data: LJL.
Performing other statistical analyses not using RevMan: LJL.
Interpreting data: LJL, HKN, AMM.
Making statistical inferences: LJL.
Writing the review: LJL.
Securing funding for the review: LJL, AMM.
Performing previous work that was the foundation of the present study: no previous work.
Serving as guarantor for the review (one review author): AMM.
Taking responsibility for reading and checking the review before submission: LJL.
Sources of support
Internal sources
Herlev University Hospital, Denmark.
External sources
No sources of support supplied
Declarations of interest
All authors: none known.
New
References
References to studies included in this review
Bakhamees 2007 {published data only (unpublished sought but not used)}
- Bakhamees HS, El‐Halafawy YM, El‐Kerdawy HM, Gouda NM, Altemyatt S. Effects of dexmedetomidine in morbidly obese patients undergoing laparoscopic gastric bypass. Middle East Journal of Anesthesiology 2007;19(3):537‐51. [PubMed] [Google Scholar]
Feld 2006 {published data only (unpublished sought but not used)}
- Feld JM, Hoffman WE, Stechert MM, Hoffman IW, Ananda RC. Fentanyl or dexmedetomidine combined with desflurane for bariatric surgery. Journal of Clinical Anesthesia 2006;18(1):24‐8. [DOI: ] [DOI] [PubMed] [Google Scholar]
Khanduja 2013 {published data only (unpublished sought but not used)}
- Khanduja S, Ohri A, Panwar M. Dexmedetomidine reduces dose requirement of thiopentone sodium and pentazocine, decreases postoperative pain and reduces haemodynamic fluctuation. Journal of Anaesthesiology Clinical Pharmacology 2014;30(2):208‐12. [DOI: 10.4103/0970-9185.130022] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mohamed 2012 {published data only (unpublished sought but not used)}
- Mohamed AA, Fares KM, Mohamed SA. Efficacy of intrathecally administered dexmedetomidine versus dexmedetomidine with fentanyl in patients undergoing major abdominal cancer surgery. Pain Physician 2012;15(4):339‐48. [PubMed] [Google Scholar]
Park 2012 {published data only (unpublished sought but not used)}
- Park J, Cheong S, Lee K, Lim S, Lee J, Cho K, et al. Does dexmedetomidine reduce postoperative pain after laparoscopic cholecystectomy with multimodal analgesia?. Korean Journal of Anesthesiology 2012;63:436‐40. [DOI: 10.4097/kjae.2012.63.5.436] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tufanogullari 2008 {published data only (unpublished sought but not used)}
- Tufanogullari B, White PF, Peixoto MP, Kianpour D, Lacour T, Griffin J, et al. Dexmedetomidine infusion during laparoscopic bariatric surgery: the effect on recovery outcome variables. Anesthesia & Analgesia 2008;106(6):1741‐8. [DOI: 10.1213/ane.0b013e318172c47c] [DOI] [PubMed] [Google Scholar]
Xiao 2013 {published data only (unpublished sought but not used)}
- Xiao C, Lu B, Yao J, Sun J. Effect of dexmedetomidine in acute postoperative pain relief is independent of suppressing the hyperalgesia induced by remifentanil. The National Medical Journal of China 2013;93(1):44‐7. [DOI: ] [PubMed] [Google Scholar]
References to studies excluded from this review
Abdalla 2003 {published data only}
- Abdalla N, Soliman AH. The effects of dexmedetomidine premedication on cortisol and interleukin‐6 in patients undergoing major abdominal surgery. Egyptian Journal of Anaesthesia 2003;19(3):283‐90. [Google Scholar]
Akinci 2011 {published data only}
- Akinci HA, Kol IO, Duger C, Kaygusuz K, Gursoy S, Mimaroglu C. Effects of the increasing doses of dexmedetomidine on the post‐anesthetic shivering. European Journal of Anaesthesiology. Conference: European Anaesthesiology Congress, EUROANAESTHESIA 2011, Amsterdam Netherlands. 2011.
Aldehayat 2011 {published data only}
- Aldehayat G. Intraoperative dexmedetomidine administration at the end of surgery prevents post anesthetic shivering. Rawal Medical Journal 2011;36:(pages not given). [Google Scholar]
Arain 2002 {published data only}
- Arain SR, Ebert TJ. The efficacy, side effects, and recovery characteristics of dexmedetomidine versus propofol when used for intraoperative sedation. Anesthesia & Analgesia 2002;95(2):461‐6. [DOI] [PubMed] [Google Scholar]
Basar 2008 {published data only}
- Basar H, Akpinar S, Doganci N, Buyukkocak U, Kaymak C, Sert O, et al. The effects of preanesthetic, single‐dose dexmedetomidine on induction, hemodynamic, and cardiovascular parameters. Journal of Clinical Anesthesia 2008;20:431‐6. [DOI] [PubMed] [Google Scholar]
Bergese 2010 {published data only}
- Bergese SD, Candiotti KA, Bokesch PM, Zura A, Wisemandle W, Bekker AY. A phase IIIb, randomized, double‐blind, placebo‐controlled, multicenter study evaluating the safety and efficacy of dexmedetomidine for sedation during awake fiberoptic intubation. American Journal of Therapeutics 2010;17:586‐95. [DOI] [PubMed] [Google Scholar]
Bhattacharjee 2010 {published data only}
- Bhattacharjee DP, Nayek SK, Dawn S, Bandopadhyay G, Gupta K. Effects of dexmedetomidine on haemodynamics in patients undergoing laparoscopic cholecystectomy ‐ A comparative study. Journal of Anaesthesiology Clinical Pharmacology 2010;26:45‐8. [Google Scholar]
Candiotti 2010 {published data only}
- Candiotti KA, Bergese SD, Bokesch PM, Feldman MA, Wisemandle W, Bekker AY. Monitored anesthesia care with dexmedetomidine: a prospective, randomized, double‐blind, multicenter trial. Anesthesia and Analgesia 2010;110:47‐56. [DOI] [PubMed] [Google Scholar]
Chen 2013 {published data only}
- Chen J, Yan J, Han X. Dexmedetomidine may benefit cognitive function after laparoscopic cholecystectomy in elderly patients. Experimental and Therapeutic Medicine 2013;5:489‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elcicek 2010 {published data only}
- Elcicek K, Tekin M, Kati I. The effects of intravenous dexmedetomidine on spinal hyperbaric ropivacaine anesthesia. Journal of Anesthesia 2010;24(4):544‐8. [DOI] [PubMed] [Google Scholar]
Gupta 2011 {published data only}
- Gupta R, Verma R, Bogra J, Kohli M, Raman R, Kushwaha JK. A comparative study of intrathecal dexmedetomidine and fentanyl as adjuvants to bupivacaine. Journal of Anaesthesiology Clinical Pharmacology 2011;27:339‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gupta 2014a {published data only}
- Gupta M, Shailaja S, Sudhir Hegde K. Comparison of intrathecal dexmedetomidine with buprenorphine as adjuvant to bupivacaine in spinal asnaesthesia. Journal of Clinical and Diagnostic Research 2014;8:114‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gupta 2014b {published data only (unpublished sought but not used)}
- Gupta P, Joshi S, Jethava D, Kumar A. Dexmedetomidine ameliorates monitored anaesthesia care. Indian Journal of Anaesthesia 2014;58(2):154‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gurbet 2006 {published data only}
- Gurbet A, Basagan‐Mogol E, Turker G, Ugun F, Kaya FN, Ozcan B. Intraoperative infusion of dexmedetomidine reduces perioperative analgesic requirements. Canadian Journal of Anaesthesia 2006;53(7):646‐52. [DOI] [PubMed] [Google Scholar]
Guryanov 2013 {published data only}
- Guryanov VA, Nosenko MM, Gedzibekov NCh, Alyautdin RN, Tolmache GN. Dexmedetomidine use for postoperative adrenergic analgesia and sedation in abdominal surgery. Anestesiologia i Reanimatologia 2013;5:21‐4. [PubMed] [Google Scholar]
Harsoor 2014 {published data only}
- Harsoor S, Rani D, Lathashree S, Nethra S, Sudheesh K. Effect of intraoperative dexmedetomidine infusion on sevoflurane requirement and blood glucose levels during entropy‐guided general anesthesia. Journal of Anaesthesiology Clinical Pharmacology 2014;30(1):25‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Iwakiri 2012 {published data only}
- Iwakiri H, Oda Y, Asada A, Ozaki M. The efficacy of continuous infusion of low dose dexmedetomidine for postoperative patients recovering in general wards. European Journal of Anaesthesiology 2012;29(5):251‐4. [DOI] [PubMed] [Google Scholar]
Jakob 2012 {published data only}
- Jakob SM, Ruokonen E, Grounds RM, Sarapohja T, Garratt C, Pocock SJ, et al. Dexmedetomidine vs midazolam or propofol for sedation during prolonged mechanical ventilation: two randomized controlled trials. JAMA 2012;307(11):1151‐60. [DOI] [PubMed] [Google Scholar]
Kaya 2010 {published data only}
- Kaya FN, Yavascaoglu B, Turker G, Yildirim A, Gurbet A, Mogol EB, Ozcan B. Intravenous dexmedetomidine, but not midazolam, prolongs bupivacaine spinal anesthesia. Canadian Journal of Anaesthesia 2010;57(1):39‐45. [DOI] [PubMed] [Google Scholar]
Keniya 2011 {published data only}
- Kenya VM, Ladi S, Naphade R. Dexmedetomidine attenuates sympathoadrenal response to tracheal intubation and reduces perioperative anaesthetic requirement. Indian Journal of Anaesthesia 2011;55:352‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lawrence 1997 {published data only}
- Lawrence CJ, Lange S. Effects of a single pre‐operative dexmedetomidine dose on isoflurane requirements and peri‐operative haemodynamic stability. Anaesthesia 1997;52(8):736‐44. [DOI] [PubMed] [Google Scholar]
Málek 2010 {published data only}
- Málek J, Marecek F, Hess L, Kurzová A, Ocadlík M, Votava M. A combination of dexmedetomidine with ketamine and opioids results in significant inhibition of hemodynamic changes associated with laparoscopic cholecystectomy and in prolongation of postoperative analgesia. Rozhledy v Chirurgii 2010;89(5):275‐81. [PubMed] [Google Scholar]
Marangoni 2005 {published data only}
- Marangoni MA, Machado Castiglia YM, Pechutti Medeiros T. Analgesic efficacy of dexmedetomidine as compared to sufentanil in intraperitoneal surgeries. Comparative study. [Eficácia analgésica da dexmedetomidina comparada ao sufentanil em cirurgias intraperitoneais. Estudo comparativo]. Revista Brasileira de Anestesiologia 2005;55(1):19‐27. [DOI] [PubMed] [Google Scholar]
No author 2007 {published data only}
- (No author reported). Intraoperative dexmedetomidine reduces perioperative morphine requirement. Journal of Pain & Palliative Care Pharmacotherapy 2007;21(1):124‐5. [Google Scholar]
Ohtani 2008 {published data only}
- Ohtani N, Kida K, Shoji K, Yasui Y, Masaki E. Recovery profiles from dexmedetomidine as a general anesthetic adjuvant in patients undergoing lower abdominal surgery. Anesthesia and Analgesia 2008;107(6):1871‐4. [DOI] [PubMed] [Google Scholar]
Ozbakis 2008 {published data only}
- Ozbakis ABC, Inanoglu K, Turhanoglu S, Asfuroglu Z. Effects of dexmedetomidine and tramadol administered before induction of anesthesia on postoperative pain. Anestezi Dergisi 2008;16:183‐7. [Google Scholar]
Padma 2013 {published data only}
- Padma L, Manasa CR, Shivshankar, Ranjani R. A randomized, open‐labelled study of the sedative, analgesic and anxiolytic effect of dexmedetomidine and tramadol in postoperative patients. International Journal of Basic & Clinical Pharmacology 2013;2(6):804‐9. [DOI: 10.5455/2319-2003.ijbcp20131225] [DOI] [Google Scholar]
Shukla 2011 {published data only}
- Shukla D, Verma A, Agarwal A, Pandey HD, Tyagi C. Comparative study of intrathecal dexmedetomidine with intrathecal magnesium sulfate used as adjuvants to bupivacaine. Journal of Anaesthesiology Clinical Pharmacology 2011;27:495‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tasdogan 2009 {published data only}
- Tasdogan M, Memis D, Sut N, Yuksel M. Results of a pilot study on the effects of propofol and dexmedetomidine on inflammatory responses and intraabdominal pressure in severe sepsis. Journal of Clinical Anesthesia 2009;21(6):394‐400. [DOI] [PubMed] [Google Scholar]
Wan 2011 {published data only}
- Wan LJ, Huang QQ, Yue JX, Lin L, Li SH. Comparison of sedative effect of dexmedetomidine and midazolam for post‐operative patients undergoing mechanical ventilation in surgical intensive care unit. Chinese Critical Care Medicine 2011;23(9):543‐6. [PubMed] [Google Scholar]
Yildiz 2006 {published data only}
- Yildiz M, Tavlan A, Tuncer S, Reisli R, Yosunkaya A, Otelcioglu S. Effect of dexmedetomidine on haemodynamic responses to laryngoscopy and intubation: perioperative haemodynamics and anaesthetic requirements. Drugs in R&D 2006;7(1):43‐52. [DOI] [PubMed] [Google Scholar]
Zeng 2014 {published data only}
- Zeng XZ, Xu YM, Cui XG, Guo YP, Li WZ. Low‐dose epidural dexmedetomidine improves thoracic epidural anaesthesia for nephrectomy. Anaesthesia and Intensive Care 2014;42(2):185‐90. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Altindis 2008 {published data only (unpublished sought but not used)}
- Altindis NT, Karaaslan D, Peker TT, Ozmen S, Bulbul M. Comparison of meperidine alone with meperidine plus dexmedetomidine for postoperative patient‐controlled analgesia. Neurosciences 2008;13:117‐21. [PubMed] [Google Scholar]
Anvaroglu 2008 {published data only (unpublished sought but not used)}
- Anvaroglu R, Kelsaka E, Sarihasan B, Demirkaya M, Ustun E, Ulger F. The effect of intravenous dexmedetomidine premedication on hemodynamic response during endotracheal intubation and extubation and postoperative analgesic consumption. Anestezi Dergisi 2008;16:201‐5. [Google Scholar]
Arain 2004 {published data only (unpublished sought but not used)}
- Arain SR, Ruehlow RM, Uhrich TD, Ebert TJ. The efficacy of dexmedetomidine versus morphine for postoperative analgesia after major inpatient surgery. Anesthesia and Analgesia 2004;98:153‐8. [DOI] [PubMed] [Google Scholar]
Bicer 2006 {published data only (unpublished sought but not used)}
- Bicer C, Esmaoglu A, Akin A, Boyaci A. Dexmedetomidine and meperidine prevent postanaesthetic shivering. European Journal of Anaesthesiology 2006 Feb;23(2):149‐53. [DOI] [PubMed] [Google Scholar]
Ceballos 2011 {published data only (unpublished sought but not used)}
- Ceballos CM, Osorio RG. Anesthesia for bariatric surgery: fentanyl versus fentanyl and dexmedetomidina [Anestesia en cirugía bariátrica: fentanil y dexmedetomidina vs. fentanil]. Neurologia Neurocirugia y Psiquiatria 2011;44:114‐21. [Google Scholar]
Kilicaslan 2006 {published data only (unpublished sought but not used)}
- Kilicaslan N, Gursoy S, Kaygusuz K, Ersecan T, Kafali H, Mimaroglu C. The effects of dexmedetomidine on stress response and desflurane requirement. Anestezi Dergisi 2006;14:237‐42. [Google Scholar]
Kordan 2006 {published data only (unpublished sought but not used)}
- Kordan A, Gunaydin B. A comparison of the effects of different doses of dexmedetomidine on the hemodynamic response to laryngoscopy and endotracheal intubation, peroperative anesthetic requirement and recovery. Anestezi Dergisi 2006;14:95‐102. [Google Scholar]
Mizrak 2010 {published data only (unpublished sought but not used)}
- Mizrak A, Koruk S, Bilgi M, Kocamer B, Erkutlu I, Ganidagli S, et al. Pretreatment with dexmedetomidine or thiopental decreases myoclonus after etomidate: a randomized, double‐blind controlled trial. Journal of Surgical Research 2010;159:e11‐e16. [DOI] [PubMed] [Google Scholar]
Scheinin 1992 {published data only (unpublished sought but not used)}
- Scheinin B, Lindgren L, Randell T, Scheinin H, Scheinin M. Dexmedetomidine attenuates sympathoadrenal responses to tracheal intubation and reduces the need for thiopentone and peroperative fentanyl. British Journal of Anaesthesia 1992;68:126‐31. [DOI] [PubMed] [Google Scholar]
Subasi 2012 {published data only (unpublished sought but not used)}
- Subasi H, Kol IO, Duger C, Kaygusuz K, Gursoy S, Mimaroglu C. Effects of dexmedetomidine and remifentanil as an adjunct of TIVA with propofol. European Journal of Anaesthesiology. Conference: European Anaesthesiology Congress, EUROANAESTHESIA 2011, Amsterdam Netherlands. 2011.
Unlugenc 2005 {published data only (unpublished sought but not used)}
- Unlugenc H, Gunduz M, Guler T, Yagmur O, Isik G. The effect of pre‐anaesthetic administration of intravenous dexmedetomidine on postoperative pain in patients receiving patient‐controlled morphine. European Journal of Anaesthesiology 2005;22(5):386‐91. [DOI: 10.1017/S0265021505000669] [DOI] [PubMed] [Google Scholar]
Yacout 2012 {published data only (unpublished sought but not used)}
- Yacout AG, Osman HA, Abdel‐Daem MH, Hammouda SA, Elsawy MM. Effect of intravenous dexmedetomidine infusion on some proinflammatory cytokines, stress hormones and recovery profile in major abdominal surgery. Alexandria Journal of Medicine 2012;48:3‐8. [DOI: 10.1016/j.aime.2011.11.001] [DOI] [Google Scholar]
Yektas 2011 {published data only (unpublished sought but not used)}
- Yektas A. Postoperative analgesic characteristics of intrathecal adjuvant agents including ketamine, fentanyl, sufentanyl, neostigmine, dexmedetomidine, midazolame and droperidole and their effects on spinal anesthesia. Regional Anesthesia and Pain Medicine. Conference: 30th Annual European Society of Regional Anaesthesia, ESRA Congress 2011, Dresden Germany. 2011.
References to ongoing studies
Awad 2014 {unpublished data only}
- Awad M. Intravenous dexmedetomidine for the prevention of postoperative shivering in patients undergoing general anesthesia. ClinicalTrials.gov.
Jung 2014 {unpublished data only}
- Jung KT. Effect of dexmedetomidine on recovery profiles of elderly patients. ClinicalTrials.gov.
Kim 2014 {unpublished data only}
- Kim YC. The effect of dexmedetomidine on postoperative analgesia. ClinicalTrials.gov.
Wai 2014 {unpublished data only}
- Multimodal Analgesic Using Morphine and COX‐2 With or Without Dexmedetomidine for Colorectal Surgery. Ongoing study May 2008.
Yoo 2014 {unpublished data only}
- Way CC. Multimodal analgesic using morphine and COX‐2 with or without dexmedetomidine for colorectal surgery. ClinicalTrials.gov.
Zeeni 2014 {unpublished data only}
- Zeeni C, Siddik S. Dexmedetomidine for postoperative analgesia after bariatric surgery. ClinicalTrials.gov.
Additional references
Barr 2013
- Barr J, Fraser GL, Puntillo K, Ely EW, Gelinas C, Dasta JF, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit, 1 ed. Critical Care Medicine 2013;41:263‐306. [DOI] [PubMed] [Google Scholar]
Biccard 2008
- Biccard BM, Goga S, Beurs J. Dexmedetomidine and cardiac protection for non‐cardiac surgery: a meta‐analysis of randomised controlled trials. Anaesthesia 2008;63(1):4‐14. [PMID: 18086064] [DOI] [PubMed] [Google Scholar]
Blaudszun 2012
- Blaudszun G, Lysakowski C, Elia N, Tramèr MR. Effect of perioperative systemic alpha2 agonists on postoperative morphine consumption and pain intensity: systematic review and meta‐analysis of randomized controlled trials. Anaesthesiology 2012;116(6):1312–22. [DOI: 10.1097/ALN.0b013e31825681cb] [DOI] [PubMed] [Google Scholar]
Bonnet 2007
- Bonnet F, Marret E. Postoperative pain management and outcome after surgery. Best Practice & Research Clinical Anaesthesiology 2007;21(1):99–107. [DOI] [PubMed] [Google Scholar]
Chan 2010
- Chan AK, Cheung CW, Chong YK. Alpha‐2 agonists in acute pain management. Expert Opinion on Pharmacotherapy 2010;11(17):2849‐68. [DOI: 10.1517/14656566.2010.511613] [DOI] [PubMed] [Google Scholar]
Cho 2014
- Cho K, Lee W, Lee J.H, Kim MH, Lee KM. Perioperative infusion of lidocaine vs. dexmedetomidine; effect on reduction of postoperative analgesics consumption after laparoscopic cholecystectomy. European Anaesthesiology Congress, EUROANAESTHESIA 2014. Stockholm Sweden: Lippincott Williams and Wilkins, May 2014, issue 31:228.
Chrysostomou 2008
- Chrysostomou C, Schmitt CG. Dexmedetomidine: sedation, analgesia and beyond. Expert Opinion on Drug Metabolism & Toxicology 2008;4(5):619‐27. [PMID: 18484919] [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. [DOI] [PubMed] [Google Scholar]
Dexdor product information
- European Medicines Agency. Dexdor product information. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Product_Information/human/002268/WC500115631.pdf. (accessed 19 October 2014).
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Greenland 1985
- Greenland S, Robins JM. Estimation of a common effect parameter from sparse follow‐up data. Biometrics 1985;41:55‐68. [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schunemann HJ. What is "quality of evidence" and why is it important to clinicians. BMJ 2008;336:995‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Ibacache 2014
- Ibacache M, Vega E, Rampinelli I, Nazar C, Elgueta F, Echevarria G. Effect of dexmedetomidine on postoperative glucose levels and insulin secretion in obese patients with impaired glucose tolerance. European Anaesthesiology Congress, EUROANAESTHESIA 2014. Stockholm Sweden: Lippincott Williams and Wilkins, May 2014, issue 31:150.
Jun 2014
- Jun GW, Kim MS, Yang HJ, Sung TY, Park DH, et al. Laparoscopic appendectomy under spinal anesthesia with dexmedetomidine infusion. Korean Society of Anesthesiologists 2014;67:246‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kehlet 2003
- Kehlet H, Dahl JB. Anaesthesia, surgery, and challenges in postoperative recovery. Lancet 2003;362(9399):1921–8. [DOI] [PubMed] [Google Scholar]
Kim JM 2014
- Kim JM, Kil HK. Randomized comparative study on the effects of epidural dexmedetomidine on heart rate variability during general anesthesia in patients undergoing gastrectomy. European Anaesthesiology Congress, EUROANAESTHESIA 2014. Stockholm Sweden: Lippincott Williams and Wilkins, May 2014:34‐35.
Le Guen 2014
- Guen M, Liu N, Tounou F, Auge M, Tuil O, et al. Dexmedetomidine reduces propofol and remifentanil requirements during bispectral index‐guided closed‐loop anesthesia: a double‐blind, placebo‐controlled trial. Anesthesia and Analgesia May 2014;118(5):946‐55. [DOI] [PubMed] [Google Scholar]
Lin 2009
- Lin TF, Yeh YC, Lin FS, Wang YP, Lin CJ, Sun WZ, et al. Effect of combining dexmedetomidine and morphine for intravenous patient‐controlled analgesia. British Journal of Anaesthesia 2009;102(1):117‐22. [PMID: 18987053] [DOI] [PubMed] [Google Scholar]
Lin 2012
- Lin YY, He B, Chen J, Wang ZN. Can dexmedetomidine be a safe and efficacious sedative agent in post‐cardiac surgery patients? A meta‐analysis. Critical Care 2012;16(5):R169. [DOI: 10.1186/cc11646] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2007
- Liu SS, Wu CL. Effect of postoperative analgesia on major postoperative complications: a systematic update of the evidence. Anesthesia and Analgesia 2007;104:689–702. [DOI] [PubMed] [Google Scholar]
Mantel 1959
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. Journal of the National Cancer Institute 1959;22:719‐48. [PubMed] [Google Scholar]
Mantz 2011
- Mantz J, Josserand J, Hamada S. Dexmedetomidine: new insights. European Journal of Anaesthesiology 2011;28(1):3‐6. [PMID: 20881501] [DOI] [PubMed] [Google Scholar]
Marret 2005
- Marret E, Kurdi O, Zufferey P, Bonnet F. Effects of nonsteroidal antiinflammatory drugs on patient‐controlled analgesia morphine side effects: meta‐analysis of randomized controlled trials. Anesthesiology 2005;102:1249–60. [DOI] [PubMed] [Google Scholar]
Naja 2014
- Naja ZM, Khatib R, Ziade FM, Moussa G, Naja ZZ, et al. Effect of clonidine versus dexmedetomidine on pain control after laparoscopic gastric sleeve: a prospective, randomized, double‐blinded study. Medknow Publications 2014;8:57‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Precedex prescribing information
- Hospira, Inc. Precedex prescribing information. http://www.precedex.com/wp‐content/uploads/wEN‐3411_PrecedexPI.PDF. (accessed 19 October 2014).
RevMan 5.3 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schnabel 2013
- Schnabel A, Meyer‐Friessem CH, Reichl SU, Zahn PK, Pogatzki‐Zahn EM. Is intraoperative dexmedetomidine a new option for postoperative pain treatment? A meta‐analysis of randomized controlled trials. Pain 2013;154(7):1140‐9. [DOI: 10.1016/j.pain.2013.03.029] [DOI] [PubMed] [Google Scholar]
Singh 2014
- Singh S, Singh V. Effect of dexmedetomidine on haemodynamics, fentanyl requirement and recovery profile in patients of laparoscopic cholecystectomy. 7th World Congress of the World Institute of Pain, WIP 2014. Maastricht Netherlands: Blackwell Publishing Inc, May 2014:95.
Tan 2010
- Tan JA, Ho KM. Use of dexmedetomidine as a sedative and analgesic agent in critically ill adult patients: a meta‐analysis. Intensive Care Medicine 2010;36(6):926‐39. [PMID: 20376429] [DOI] [PubMed] [Google Scholar]
Vandermeulen 2006
- Vandermeulen E. Systemic analgesia and co‐analgesia. Acta Anaesthesiologica Belgica 2006;57(2):113‐20. [PubMed] [Google Scholar]
Villela 2003
- Villela NR, Nascimento Júnior P. Dexmedetomidine in anesthesiology [in Portuguese]. Revista Brasileira de Anestesiologia 2003;53(1):97‐113. [PMID: 19475263] [DOI] [PubMed] [Google Scholar]
Wang 2015
- Wang Y, Xu X, Liu H, Ji F. Effects of dexmedetomidine on patients undergoing radical gastrectomy. The Journal of Surgical Research 2015;194:147‐53. [DOI] [PubMed] [Google Scholar]
White 2010
- White PF, Kehlet H. Improving postoperative pain management: what are the unresolved issues?. Anesthesiology 2010;112(1):220‐5. [DOI] [PubMed] [Google Scholar]
Wijeysundera 2009
- Wijeysundera DN, Bender JS, Beattie WS. Alpha‐2 adrenergic agonists for the prevention of cardiac complications among patients undergoing surgery. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD004126.pub2] [DOI] [PubMed] [Google Scholar]
Ziemann‐Gimmel 2014
- Ziemann‐Gimmel P, Goldfarb AA, Koppman J, Marema RT. Opioid‐free total intravenous anaesthesia reduces postoperative nausea and vomiting in bariatric surgery beyond triple prophylaxis. British Journal of Anaesthesia 2014;112:906‐11. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Jessen 2013
- Jessen Lundorf L, Korvenius Jørgensen H, Møller AM. Perioperative dexmedetomidine for acute pain after abdominal surgery in adults. Cochrane Database of Systematic Reviews 2013, Issue 2. [DOI: 10.1002/14651858.CD010358] [DOI] [PMC free article] [PubMed] [Google Scholar]


