Abstract
Radiation-induced dermatitis is a common occurrence in cancer patients undergoing radiation therapy (RT) and is caused when ionizing radiation (IR) induces DNA strand breaks in skin cells. The wide use of RT in cancer treatments makes it important to minimize RT-induced toxicities including radiodermatitis. This study sought to determine if the circadian clock plays a protective role in minimizing radiodermatitis. We treated mice in control (Day Shift), environmentally-disrupted (Rotating Shift) and genetically-disrupted (Per 1/2−/−) circadian conditions with 6 Gy of IR to the whole body. There was a significantly increased number of radiodermatitis spots on mice with circadian clock disruption compared to control mice. Additionally, circadian clock disrupted mice exhibited reduced protein levels of Bmal1, a phenomenon that sensitized circadian synchronized keratinocytes to IR-induced DNA damage. Furthermore, the skin phenotype results corresponded with significantly reduced body weights and increased genomic DNA damage in blood cells of mice with clock disruption compared to control mice. These findings suggest that the circadian clock plays a protective role in IR-induced DNA damage and skin toxicity, possibly through BMAL1-dependent mechanisms, and potentially impacts RT-associated radiodermatitis in cancer patients.
Keywords: Circadian rhythm, radiodermatitis, toxicity, DNA damage, BMAL1
INTRODUCTION
Radiodermatitis is a common side effect experienced by up to 95% of patients undergoing radiation therapy (RT) (McQuestion, 2011). This condition is characterized by pain, redness, itchiness, and lesions, which decrease the patients’ quality of life and survival (Fitzgerald et al., 2008). RT utilizes ionizing radiation (IR) to induce DNA strand breaks within tumors to cause efficient cell death, but unfortunately the skin is inadvertently affected (Fitzgerald et al., 2008). Given that RT remains an essential part of cancer treatment programs, with over 6000 active clinical trial studies as of this writing (National Library of Medicine (U.S.), 2019), it is therefore important to understand mechanisms that mitigate RT-associated radiodermatitis.
Here, we investigate the role the endogenous circadian clock may play in limiting radiodermatitis. The circadian (~24 hour) clock of mammals is a time-keeping machinery that persists from molecules to physiology (Shearman et al., 2000). At the molecular level, a circadian oscillation is generated primarily by transcriptional-translational feedback loops by core positive (Clock-Bmal1) and negative (Cryptochrome (Cry1–2) and Period (Per1–3)) elements which drive the expression of many target clock-controlled genes (CCGs) (Shearman et al., 2000; Partch et al., 2014). Additional regulation occurs through the secondary arm elements, Rorα and Rev-erbα, that activate and inhibit Arntl (Bmal1) respectively (Cho et al., 2012).
Robust rhythmicity of the core molecular oscillator is known to be present in mouse and human skin, and cell proliferation and DNA repair have been shown to be controlled by components of the molecular clockwork. In addition, it was demonstrated that damage to DNA by UVB exposure as well as repair varied by time-of-day, reinforcing the importance of the molecular clock in maintaining homeostasis of mouse and human skin (Gaddameedhi et al., 2011; Geyfman et al., 2012; Plikus et al., 2013; Gaddameedhi et al., 2015; Dakup et al., 2018; Nikkola et al., 2018; Wu et al., 2018).
As molecular evidence has shown the alteration of the core clock components in skin dysregulates the cellular processes involved in skin homeostasis (Geyfman et al., 2012; Gaddameedhi et al., 2015), we hypothesized that the same concept could be applied to RT in order to understand how circadian disruption impacts radiotoxicity including radiodermatitis. We carried out our studies using the SKH-1 hairless mouse model in which the clock was disrupted genetically (Per1/2−/−) or environmentally (Rotating Shift), alongside undisrupted controls (Day Shift).
MATERIALS AND METHODS
Animal housing and clock-disruption protocols
All animal procedures were in accordance with the National Institutes of Health guidelines and approved by the Institutional Animal Care and Use Committee of Washington State University. Wild-type SKH-1 hairless mice (4- to 5-week old, female) were obtained from Charles River Laboratories. The circadian clock-disrupted Per1, Per2 mutant mice in the SKH-1 genetic background were developed in our lab by backcrossing for at least 12 generations with Per1, Per2 mutant mice in the C57BL/6 genetic background, which were obtained from the Jackson Laboratory and originally developed and characterized by Dr. David Weaver’s group (Bae et al., 2001). These Per1/2−/− mice have specific exon deletions in Per1 and Per2 gene sequences so there is no interaction with the bHLH and PAS domains of the CLOCK-BMAL1 heterodimeric complex during transcription and thus confer circadian disruption. Otherwise, these mice are healthy and viable for our studies. Day Shift (controls) and Per1/2−/− mice were maintained under a 12-hour light/12-hour dark (LD 12:12) cycle (lights on at 7 AM, ZT0, and lights off at 7 PM, ZT12) at least 4 weeks before and throughout the duration of the study. ZT means Zeitgeber Time where a zeitgeber is the cue that resets circadian rhythms, which in this case is light. Our rotating shift protocol of weekly alternating light/dark cycles was adopted from previously published work and was initiated 15 days prior to RT (Van Dycke et al., 2015).
Irradiation treatments and toxicity monitoring
Total body irradiation (TBI) was performed using an X-RAD 320 irradiator (Precision X-Ray, Inc). The dosage of 6 Gy was chosen based on the range of TBI for leukemia patients (Wong et al., 2018). Conscious SKH-1 mice were treated with whole body irradiation in a well-ventilated 21.5 cm pie chamber. The dose output was 0.5 Gy/min using a 1.5 mm Al, 0.25 mm Cu, 0.75 mm Sn filter for a total dose of 6 Gy. Body weights were measured post-irradiation for all mice using an analytical balance. Acute dermatitis was evaluated in irradiated mice by visual inspection of the skin for the physical appearance of small circular reddish spots as previously described (Jang et al., 2018). Treatments and tissue harvesting were done between ZT 11 and 12, for rotating shift mice, these occurred 1 day into a new LD cycle so their body clock would not have had enough time to entrain to the new cycle.
Comet assay
Blood samples were collected from mice by cardiac puncture, immediately added to RPMI media + 20% DMSO (1:1 v/v), and stored at −80°C. Comet assay was performed as previously described (Townsend et al., 2017). Normal melting point agarose (1% w/v) was prepared in MilliQ water, microwaved until in solution and kept in a 37°C water bath to prevent solidification. One ml of agarose was pipetted onto clear microscope slides, covered with coverslips, and allowed to solidify for 15 minutes. Following solidification, coverslips were removed, and slides were dried at room temperature overnight. Lymphocytes were isolated by performing two red blood cell (RBC) lysis steps, each accomplished by adding 500 μl of RBC lysis buffer (0.15 M NH4Cl, 0.01 M NaHCO3, 1 mM EDTA) to previously stored blood samples, incubating for 5 minutes and centrifuging at 1500 RCF for 1 minute. Lymphocytes were then washed twice in 1x PBS and centrifuged at 12000 RCF for 1 minute at 4°C. Low melting point agarose (0.6%) (LMA) was prepared in PBS, microwaved until in solution, and kept in a 40°C water bath to prevent solidification. LMA cell suspensions were prepared by briefly vortexing cell pellets to disperse cells and adding 210 μl of LMA followed by pipetting to mix. The cell suspension (100 μl) was pipetted onto each of two slides for each sample, covered with a coverslip, and placed in a 4°C cold room for 30 minutes to ensure solidification. Coverslips were then removed and slides were placed in alkaline lysis buffer (100 mM EDTA, 2.5 M NaCl, 10 mM Tris, pH 10) allowing agarose embedded cells to lyse overnight at 4°C. Following lysis, slides were washed for 30 minutes with cold MilliQ water, transferred to an electrophoresis tank (Cleaver Scientific), submerged in chilled alkaline electrophoresis buffer (300 mM NaOH, 1 mM EDTA), and allowed to incubate for 30 minutes. Electrophoresis was then performed at 21 V for 50 minutes. Slides were then drained of electrophoresis buffer, incubated in chilled neutralization buffer (0.4 M Tris, pH 7.5) followed by chilled MilliQ water for 20 minutes each, then placed in an unhumidified 37°C incubator to dry overnight. For imaging, slides were removed from the incubator, allowed to rehydrate in MilliQ water for 20 minutes, stained for 20 minutes with 2.5 μg/μl propidium iodide solution (Sigma-Aldrich P4170), and washed again for 20 minutes in MilliQ water. Images were captured using an Axio Observer Z1 Microscope (Leica) at 20x magnification and a random sample of 50 cells was selected for each animal and scored using CometScore software (TriTek) to determine the average percentage of DNA contained in the comet tails.
Cell culture Studies
Human keratinocytes (HaCaT) (a gift from Dr. Robert Smart of NC State University) (Ming et al., 2011) were cultured in DMEM supplemented with 10% FBS and circadian synchronized by serum shock as previously described to mimic circadian condition in vitro (Balsalobre et al., 1998). Briefly, cells were grown to approximately 100% confluency, at which point DMEM supplemented with 10% FBS was replaced with DMEM supplemented with 50% FBS and kept in a 37°C incubator with 5% CO2 for two hours. Next, cells were gently washed twice with 1x PBS, and further incubated with serum-free DMEM containing either vehicle control (DMSO) or 25 μM Rev-erbα agonist SR9011 (Cayman chemical; Cat # 1930). This agonist inhibits Bmal1 while not affecting normal cell viability (Trump et al., 2013; Sulli et al., 2018). Cells were further treated with 6 Gy of irradiation at a rate of 1.3 Gy/min using a 2 mm Al filter and then incubated further until the collection timepoints at 8, 24, and 48 hours post-irradiation.
Immunoblotting
Frozen skin tissue samples were homogenized in liquid nitrogen using a mortar and pestle. Protein lysate was extracted from tissues using 1x RIPA lysis buffer (20 mM Tris-HCl pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Nonidet P-40, and 1% sodium deoxycholate) plus 1x protease inhibitors (Fisher Scientific; Cat # 88666), and from cells using 1x lysis buffer (50 mM Tris-HCl pH 7.5, 150 mM NaCl, 1 mM EDTA, 1% Tween-20) plus protease inhibitors and to determine the protein levels, the immunoblotting protocol was followed as previously described by us (Gaddameedhi et al., 2011; Gaddameedhi et al., 2015). The following antibodies were used: GAPDH (Santa Cruz Biotechnology; Cat # sc-25778), BMAL1 (Bethyl Laboratories; Cat # A302–616A), β-Tubulin and pH2A.X (Cell Signaling Technology; Cat #s 86298T and 9718S, respectively). The appropriate HRP-conjugated secondary antibody was used for detection with chemiluminescence (Clarity Western ECL, Bio-Rad, and/or SuperSignal West Femto, Thermo Fisher Scientific) using a Bio-Rad ChemiDoc XRS+ imager.
Quantification and statistics
All results are represented as Mean ± SEM. Two-tailed student’s t-tests and two-way ANOVA (with repeated measures for time-course analysis) were performed using Prism version 6.01 (GraphPad software). Dunnett’s multiple comparison tests were used for post-hoc comparisons to control where appropriate.
RESULTS
The circadian clock protects SKH-1 mice from developing acute radiodermatitis
To investigate how the circadian clock could modulate acute radiodermatitis, we designed our study as outlined in Fig. 1a using SKH-1 hairless mice. Small red radiodermatitis spots were observed on the skin of SKH-1 hairless mice 8 days post-irradiation (Fig. 1b). After scoring radiodermatitis spots over the entire body, a consistent increase in occurrence was found from day 8 to 13 in the clock-disrupted groups relative to the Day Shift group (Fig. S1). Analysis of acute radiodermatitis spots on day 13 showed a significant increase in Rotating Shift and Per1/2−/− mice compared to Day Shift (Fig. 1c).
Fig. 1. The circadian clock protects against acute radiodermatitis.
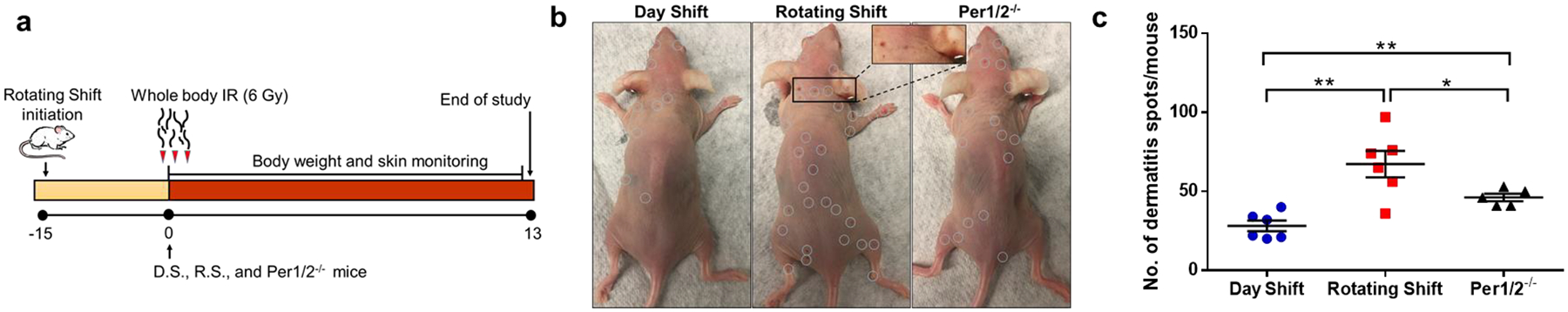
SKH-1 hairless female mice (8- to 12-week old) were used in all groups. Day Shift (D.S.) serves as control, while Rotating Shift (R.S.) and Per1/2−/− represent the environmentally- and genetically-disrupted clock conditions. a) The study design showing wild-type mice placed in Rotating Shift, characterized by weekly alternating Light/Dark cycles, for 15 days prior to IR treatment and through the duration of the study. Mice received a 6 Gy single dose of total body IR on Day 0. b) Dermatitis (red) spots (circled white) on mice skin were carefully observed and counted across the body post-IR c) Dot plot showing the number of dermatitis spots per mouse counted on Day 13 post-IR. Statistical comparison was done using Student’s t test *=p<0.05. **=p<0.01. Error bars = S.E.M. (n=5–6 mice per group).
Reduction in BMAL1 sensitizes the skin to DNA damage
It has been shown that Bmal1, a component of the positive arm of the canonical molecular circadian clock, regulates genes involved in DNA damage response (DDR) signaling, such as p.H2A.X and Xpa (Geyfman et al., 2012; Dakup et al., 2018). Hence, we sought to understand how clock disruption impacts Bmal1 in the skin. Significant decreases in the protein expression of Bmal1 were found in clock disrupted mice as compared to untreated control mice (Fig. 2a–b). These results suggest Bmal1 may provide protection from radiation-induced damage. To further understand the role of Bmal1 in radioprotection on a cellular level, we used circadian synchronized human keratinocytes (HaCaT), which exhibit robust circadian rhythms (Sporl et al., 2011). We exposed cells to 6 Gy of IR following treatment with Rev-erbα agonist SR9011, to inhibit Bmal1, or DMSO control (Trump et al., 2013). We observed increased DNA damage levels, by H2a.x phosphorylation, when Bmal1 is inhibited, with a maximum effect seen at 48 hours post-IR compared to DMSO controls (Fig. 2c–d). In the DMSO control cells, we see damage is only very slightly increased post-IR indicating protection from induction or repair of DNA damage, whereas the BMAL1-deficient cells have increased levels of DNA damage, especially at 48 hours. Taken together, these data suggest that protection of the skin from IR-induced damage might be dependent on Bmal1-related mechanisms.
Fig. 2. Reduced Bmal1 protein levels with clock disruption sensitize the skin to ionizing radiation.
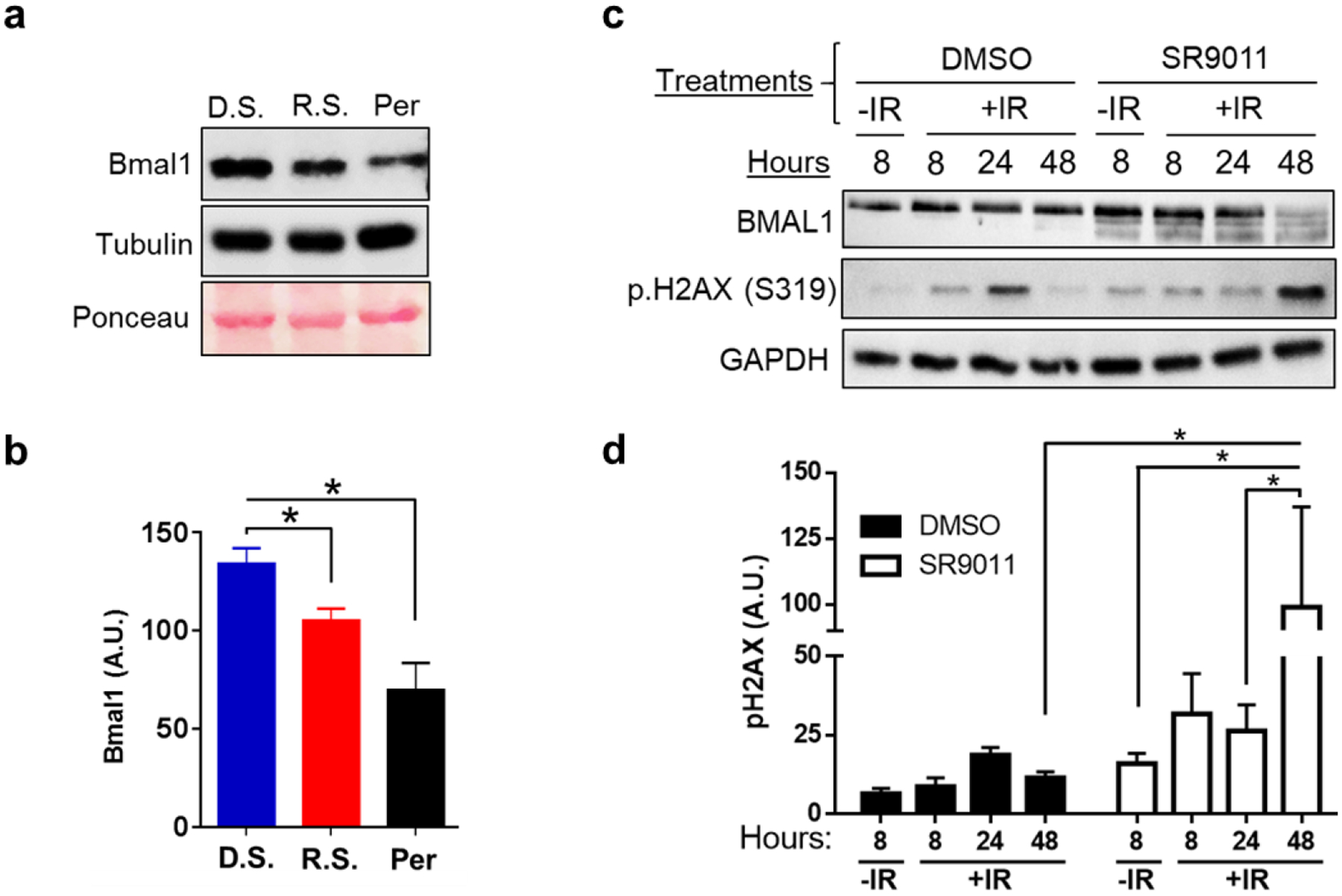
. a) Protein lysates from untreated whole skin tissue samples were probed by immunoblotting for Bmal1 expression. Densitometry analysis of immunoblot images normalized to β-Tubulin is shown in b. D.S. means Day Shift, R.S. means Rotating Shift, and Per means Per1/2−/−. Statistical analysis was done using student’s t-test. c) Human keratinocyte (HaCaT) cells were synchronized, treated with Rev-Erbα agonist (25 μM SR9011) or DMSO control, then cells were irradiated with 6 Gy of IR (with untreated negative control). Cells were collected at 8, 24, and 48 hours post-IR and protein lysates were probed by immunoblotting for Bmal1 and p.H2ax. Densitometry analysis of immunoblot images normalized to Gapdh is shown in d. Statistical analysis was done using Two-way ANOVA. For all analysis, *=p<0.05. Error bars = S.E.M. (n=3).
The circadian clock protects from systemic acute radiation-induced toxicity
Because our design employed the use of total body irradiation (TBI), we sought to understand global radiotoxicity beyond the skin. We utilized whole body weight loss as a measure of toxicity and found that Day Shift mice displayed a better tolerance to RT relative to the clock-disrupted mice (Fig. 3a). The weight of Day Shift mice only decreased slightly, by up to 3.5% at day 6, after which body weight recovered with up to 6.5% weight gain by day 10. In the Rotating Shift group, body weight was decreased by up to 8% on day 4 after which body weight recovered with up to a maximum of 12% weight gain. In the Per1/2−/− group, body weight loss was observed through day 12, with up to a 14% weight loss, notwithstanding a slight recovery on day 6, which did not alter the trajectory of the weight loss. Compared to the Day Shift control group, there was a significant weight loss in the Rotating Shift and Per1/2−/− groups on day 4. On day 13, all mice were sacrificed due to continuous body weight loss by the Per1/2−/− mice. The rapid recovery of body weight specifically in the Rotating Shift model was interesting, and we speculate that this group shifted their clock with IR treatment, as a similar trend is seen in the Day Shift control group but not in the Per1/2−/− group (Oklejewicz et al., 2008). Blood samples were subsequently analyzed using the alkaline comet assay to assess cellular DNA damage. This analysis revealed significantly higher incidence of genomic DNA damage (comet tail) at 2 weeks post-irradiation in both the clock-disrupted groups compared to Day Shift control (Figs. 3b–c).
Fig. 3. The circadian clock protects from systemic acute IR-induced toxicity.
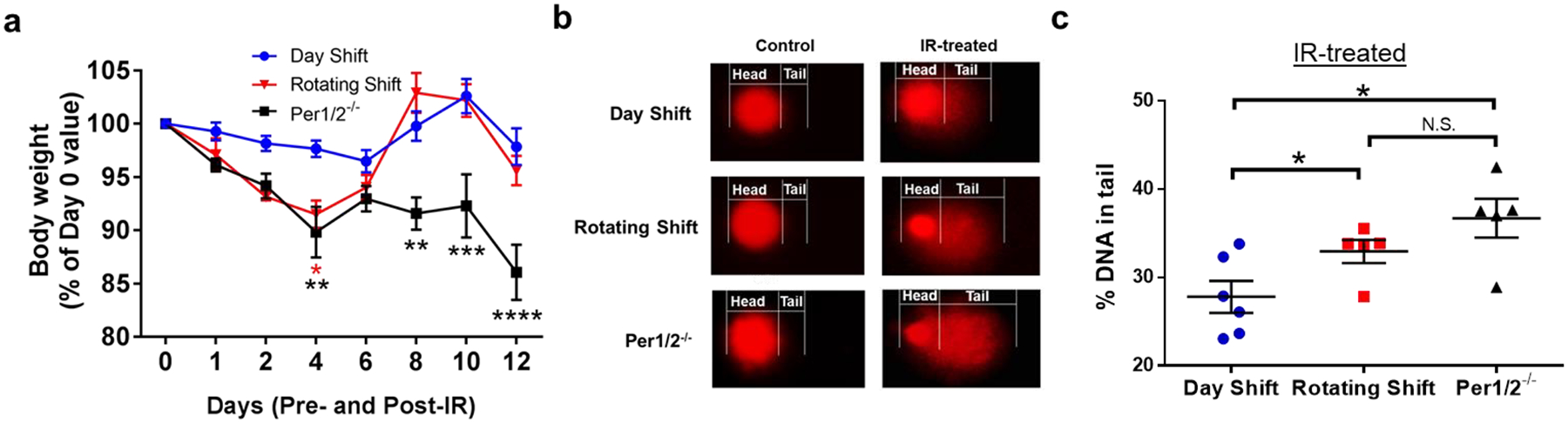
. a) Body weights were measured pre- (day 0) and post-IR (days 1–12). b) Representative images of alkaline comet assay to detect DNA strand breaks from SKH-1 mouse blood cells in control and IR-treated mice collected on day 13. c) Quantification of genomic DNA as % DNA in tail. Repeated Measures Two-way ANOVA and two-tailed student’s t-tests were used to compare body weights and comet assay analysis respectively to control/D.S. group *=p<0.05. **=p<0.01, ***=p<0.001, ****=p<0.0001. Error bars = S.E.M. N.S. means not significant. (n=5–6 mice per group).
DISCUSSION
Our results demonstrate that there is increased radiodermatitis and genotoxicity of IR in clock disrupted groups, which may stem from the cascade of cellular events through the circadian clock, including cell cycle arrest, DNA repair, and apoptosis (Plikus et al., 2013; Sancar et al., 2015). While our recent study showed the circadian clock’s protection against IR-induced cardiotoxicity through Bmal1-dependent DDR signaling mechanisms (Dakup et al., 2020), very little is known about how the circadian clock could protect IR-exposed skin from dermatitis.
The skin utilizes DDR signaling including DNA repair mechanisms to maintain cellular and genomic integrity against genotoxic stress. The influence of the molecular clock on these mechanisms has been demonstrated in various modalities over the years; from the mouse hair follicle, where loss of Cry1/2 abolishes the G2/M checkpoint response (Plikus et al., 2013); to human cells where Per2 complexes with p53 and Mdm2 and must dissociate to allow for cell cycle arrest and repair (Gotoh et al., 2015); to mouse liver, where γ radiation induces the expression of many canonical clock genes and where Per2 mutation reduces p53 expression (Fu et al., 2002). These studies, paired with therapeutic relevance, focused our studies on IR. Treatments were administered between ZT11 and ZT12 when skin cells are in early S-phase (the phase of reduced sensitivity to IR and better repair of IR damage) (Plikus et al., 2013). The Rotating Shift mice were 1 day into a new LD cycle, so their body clock times were shifted without opportunity for recovery at the point of treatment. Our models showed decreased expression of Bmal1 in the skin at the time of IR treatment (Fig. 2a–b), similar to previous findings in mouse heart (Dakup et al., 2020). Further, Bmal1 has roles as both a transcription factor for Period genes, and as a critical regulator of the cell cycle inhibitor p21WAF1/CIP1 (Grechez-Cassiau et al., 2008). Collectively, we believe that the decrease in Bmal1 due to clock disruption represses transcriptional activation of the Per2-p53 complex and p21, thus increasing radiosensitivity. Future studies are still needed to fully elucidate the role of Bmal1 and Bmal1-associated mechanisms in modulating the skin’s response to IR damage.
While genetic modification of the canonical clock has been established to study the molecular components of the clock, it is particularly important to understand the contribution of environmental disruption because internal circadian rhythms are desynchronized with modern living conditions such as shift work, which affects over 21 million workers in the United States (McMenamin, 2007). A few studies have used rotating shift and jet lag-based clock disruption models to understand metabolic disorders and carcinogenesis outcomes, but none have investigated the implications of environmental clock disruption from a therapeutic angle (Van Dycke et al., 2015; Papagiannakopoulos et al., 2016). In bridging this gap, our environmental clock disruption approach seems to result in similar measures of radiodermatitis and DNA damage as genetic disruption (Figs. 1 & 3), hinting that translating the impact of clock disruption from rodents to humans may be clinically relevant. While our data implies that Bmal1 might protect against radiodermatitis, multiple other factors resulting from environmental disruption such as hormone secretion, metabolism, and inflammatory responses could contribute to our observation (Dauchy et al., 2014; Papagiannakopoulos et al., 2016). These findings may contribute to the paradigm shift towards personalized medicine, which seeks to integrate key circadian and tumor-related biomarkers to optimize therapeutic outcome for individual patients.
A myriad of factors must be considered when proposing personalized medicine, including genetic, lifestyle, and environmental differences. Among these, sex differences are very important, with a clinical report on bone metastasis patients showing that women had a better response to RT when treated in the mid-day unlike the men who had no time-of-day-dependent response (Chan et al., 2017). This finding also hints at the importance of the circadian clock in personalized medicine generally, as well as in determining the time-of-day differences of RT response seen in women specifically. Consequently, our study employed the use of female mice to understand the impact of clock disruption in RT. Additionally, RT is used to treat most breast cancers, a cancer type with an increased risk among night shift working women (Megdal et al., 2005). Another consideration is sleep-wake timing, which is tied to the circadian clock, as well as various hormone levels. One hormone that is impacted by light to regulate the sleep/wake cycle is melatonin. Melatonin has been shown to have protective roles against breast tumor development and radiation damage to the skin (Dauchy et al., 2014; Slominski et al., 2018). However, our mouse models do not produce melatonin (data not shown) so our study is able to investigate the radioprotective effects of the circadian clock independent of melatonin. Therefore, our study lays a foundation in understanding the role of the clock in time-of-day based RT regimens for further studies using tumor models for clinical applications.
In conclusion, our findings suggest that the circadian clock regulation of DDR signaling mechanisms is a major contributor to radiotoxicity, such as radiodermatitis. Alongside available clinical data, the manipulation of the circadian clock can potentially be applied to inform the management of radiation-induced toxicities in the skin, and across different tissues of the body.
Supplementary Material
Acknowledgements/funding:
We thank Drs. Michael G. Kemp, William K. Kaufmann, and Bala Koritala for providing helpful comments. This work was supported by grants from the National Institutes of Health CA227381, ES030113 (to S.G.) and in part by the Congressionally Directed Medical Research Program Award CA171123 (to S.G.); the American Heart Association Pre-doctoral Fellowship (to P.D.).
Footnotes
Conflict of Interest Disclosure: The authors declare that they have no conflicts of interest.
References
- Bae K, Jin X, Maywood ES, Hastings MH, Reppert SM, Weaver DR, 2001. Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron 30, 525–536. [DOI] [PubMed] [Google Scholar]
- Balsalobre A, Damiola F, Schibler U, 1998. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell 93, 929–937. [DOI] [PubMed] [Google Scholar]
- Chan S, Zhang L, Rowbottom L, McDonald R, Bjarnason GA, Tsao M, Barnes E, Danjoux C, Popovic M, Lam H, DeAngelis C, Chow E, 2017. Effects of circadian rhythms and treatment times on the response of radiotherapy for painful bone metastases. Ann Palliat Med 6, 14–25. [DOI] [PubMed] [Google Scholar]
- Cho H, Zhao X, Hatori M, Yu RT, Barish GD, Lam MT, Chong LW, DiTacchio L, Atkins AR, Glass CK, Liddle C, Auwerx J, Downes M, Panda S, Evans RM, 2012. Regulation of circadian behaviour and metabolism by REV-ERB-alpha and REV-ERB-beta. Nature 485, 123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakup PP, Porter KI, Gajula RP, Goel PN, Cheng Z, Gaddameedhi S, 2020. The circadian clock protects against ionizing radiation-induced cardiotoxicity. FASEB J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakup PP, Porter KI, Little AA, Gajula RP, Zhang H, Skornyakov E, Kemp MG, Van Dongen HPA, Gaddameedhi S, 2018. The circadian clock regulates cisplatin-induced toxicity and tumor regression in melanoma mouse and human models. Oncotarget 9, 14524–14538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauchy RT, Xiang S, Mao L, Brimer S, Wren MA, Yuan L, Anbalagan M, Hauch A, Frasch T, Rowan BG, Blask DE, Hill SM, 2014. Circadian and melatonin disruption by exposure to light at night drives intrinsic resistance to tamoxifen therapy in breast cancer. Cancer Res 74, 4099–4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald TJ, Jodoin MB, Tillman G, Aronowitz J, Pieters R, Balducci S, Meyer J, Cicchetti MG, Kadish S, McCauley S, Sawicka J, Urie M, Lo YC, Mayo C, Ulin K, Ding L, Britton M, Huang J, Arous E, 2008. Radiation therapy toxicity to the skin. Dermatol Clin 26, 161–172, ix. [DOI] [PubMed] [Google Scholar]
- Fu L, Pelicano H, Liu J, Huang P, Lee C, 2002. The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell 111, 41–50. [DOI] [PubMed] [Google Scholar]
- Gaddameedhi S, Selby CP, Kaufmann WK, Smart RC, Sancar A, 2011. Control of skin cancer by the circadian rhythm. Proc Natl Acad Sci U S A 108, 18790–18795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaddameedhi S, Selby CP, Kemp MG, Ye R, Sancar A, 2015. The circadian clock controls sunburn apoptosis and erythema in mouse skin. J Invest Dermatol 135, 1119–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyfman M, Kumar V, Liu Q, Ruiz R, Gordon W, Espitia F, Cam E, Millar SE, Smyth P, Ihler A, Takahashi JS, Andersen B, 2012. Brain and muscle Arnt-like protein-1 (BMAL1) controls circadian cell proliferation and susceptibility to UVB-induced DNA damage in the epidermis. Proc Natl Acad Sci U S A 109, 11758–11763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh T, Vila-Caballer M, Liu J, Schiffhauer S, Finkielstein CV, 2015. Association of the circadian factor Period 2 to p53 influences p53’s function in DNA-damage signaling. Mol Biol Cell 26, 359–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grechez-Cassiau A, Rayet B, Guillaumond F, Teboul M, Delaunay F, 2008. The circadian clock component BMAL1 is a critical regulator of p21WAF1/CIP1 expression and hepatocyte proliferation. J Biol Chem 283, 4535–4542. [DOI] [PubMed] [Google Scholar]
- Jang H, Myung H, Lee J, Myung JK, Jang WS, Lee SJ, Bae CH, Kim H, Park S, Shim S, 2018. Impaired Skin Barrier Due to Sebaceous Gland Atrophy in the Latent Stage of Radiation-Induced Skin Injury: Application of Non-Invasive Diagnostic Methods. Int J Mol Sci 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMenamin TM, 2007. A time to work: recent trends in shift work and flexible schedules. Monthly Labor Review, 1–15. [Google Scholar]
- McQuestion M, 2011. Evidence-based skin care management in radiation therapy: clinical update. Semin Oncol Nurs 27, e1–17. [DOI] [PubMed] [Google Scholar]
- Megdal SP, Kroenke CH, Laden F, Pukkala E, Schernhammer ES, 2005. Night work and breast cancer risk: a systematic review and meta-analysis. Eur J Cancer 41, 2023–2032. [DOI] [PubMed] [Google Scholar]
- Ming M, Feng L, Shea CR, Soltani K, Zhao B, Han W, Smart RC, Trempus CS, He YY, 2011. PTEN positively regulates UVB-induced DNA damage repair. Cancer Res 71, 5287–5295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Library of Medicine (U.S.), 2019. ClinicalTrials.gov linking patients to medical research. U.S. National Library of Medicine, National Institutes of Health, Dept. of Health & Human Services,, Bethesda, MD, pp. [Google Scholar]
- Nikkola V, Gronroos M, Huotari-Orava R, Kautiainen H, Ylianttila L, Karppinen T, Partonen T, Snellman E, 2018. Circadian Time Effects on NB-UVB-Induced Erythema in Human Skin In Vivo. J Invest Dermatol 138, 464–467. [DOI] [PubMed] [Google Scholar]
- Oklejewicz M, Destici E, Tamanini F, Hut RA, Janssens R, van der Horst GT, 2008. Phase resetting of the mammalian circadian clock by DNA damage. Curr Biol 18, 286–291. [DOI] [PubMed] [Google Scholar]
- Papagiannakopoulos T, Bauer MR, Davidson SM, Heimann M, Subbaraj L, Bhutkar A, Bartlebaugh J, Vander Heiden MG, Jacks T, 2016. Circadian Rhythm Disruption Promotes Lung Tumorigenesis. Cell Metab 24, 324–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partch CL, Green CB, Takahashi JS, 2014. Molecular architecture of the mammalian circadian clock. Trends Cell Biol 24, 90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plikus MV, Vollmers C, de la Cruz D, Chaix A, Ramos R, Panda S, Chuong CM, 2013. Local circadian clock gates cell cycle progression of transient amplifying cells during regenerative hair cycling. Proc Natl Acad Sci U S A 110, E2106–2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A, Lindsey-Boltz LA, Gaddameedhi S, Selby CP, Ye R, Chiou YY, Kemp MG, Hu J, Lee JH, Ozturk N, 2015. Circadian clock, cancer, and chemotherapy. Biochemistry 54, 110–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearman LP, Sriram S, Weaver DR, Maywood ES, Chaves I, Zheng B, Kume K, Lee CC, van der Horst GT, Hastings MH, Reppert SM, 2000. Interacting molecular loops in the mammalian circadian clock. Science 288, 1013–1019. [DOI] [PubMed] [Google Scholar]
- Slominski AT, Hardeland R, Zmijewski MA, Slominski RM, Reiter RJ, Paus R, 2018. Melatonin: A Cutaneous Perspective on its Production, Metabolism, and Functions. J Invest Dermatol 138, 490–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporl F, Schellenberg K, Blatt T, Wenck H, Wittern KP, Schrader A, Kramer A, 2011. A circadian clock in HaCaT keratinocytes. J Invest Dermatol 131, 338–348. [DOI] [PubMed] [Google Scholar]
- Townsend TA, Parrish MC, Engelward BP, Manjanatha MG, 2017. The development and validation of EpiComet-Chip, a modified high-throughput comet assay for the assessment of DNA methylation status. Environ Mol Mutagen 58, 508–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trump RP, Bresciani S, Cooper AW, Tellam JP, Wojno J, Blaikley J, Orband-Miller LA, Kashatus JA, Boudjelal M, Dawson HC, Loudon A, Ray D, Grant D, Farrow SN, Willson TM, Tomkinson NC, 2013. Optimized chemical probes for REV-ERBalpha. J Med Chem 56, 4729–4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dycke KC, Rodenburg W, van Oostrom CT, van Kerkhof LW, Pennings JL, Roenneberg T, van Steeg H, van der Horst GT, 2015. Chronically Alternating Light Cycles Increase Breast Cancer Risk in Mice. Curr Biol 25, 1932–1937. [DOI] [PubMed] [Google Scholar]
- Wu G, Ruben MD, Schmidt RE, Francey LJ, Smith DF, Anafi RC, Hughey JJ, Tasseff R, Sherrill JD, Oblong JE, Mills KJ, Hogenesch JB, 2018. Population-level rhythms in human skin with implications for circadian medicine. Proc Natl Acad Sci U S A 115, 12313–12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


