Fig. 4.
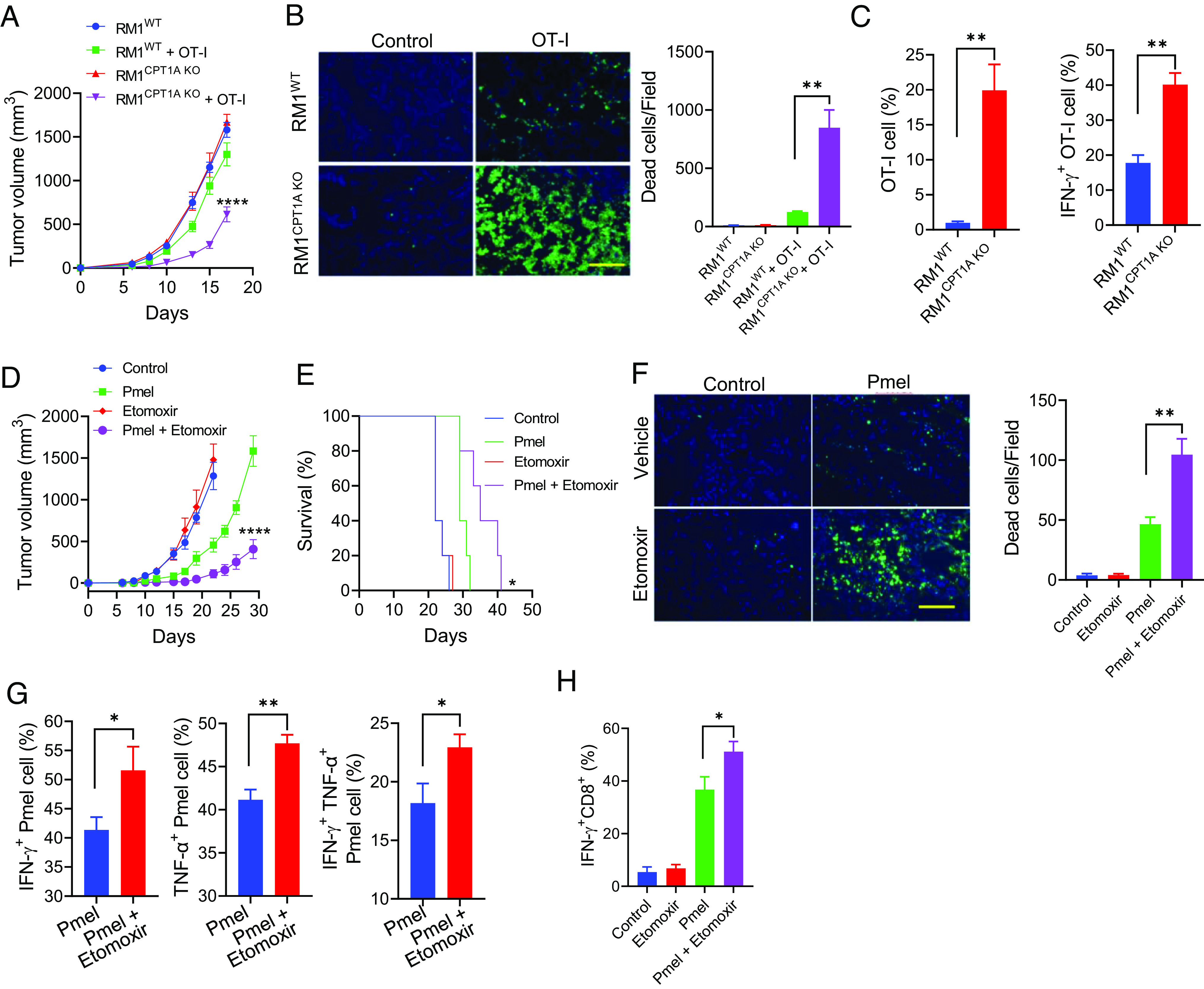
Pharmaceutical inhibition of FAO improves the antitumor efficacy of T cell therapy. Male mice with OVA-expressing RM1WT or RM1CPT1A KO prostate tumors received OT-I cells on days 2, 4, and 7 after tumor implantation (A). Prostate tumor-bearing mice (n = 5) received OT-I cells on days 9 and 12 following tumor implantation. Tumor tissues were collected 2 d after treatment for TUNEL assays (B). Infiltration and activation of OT-I cells in the tumors were assessed (C). Mice (n = 5) with B16WT or B16CPT1A KO melanomas were treated with Pmel T cells on days 4 and 8 post-tumor implantation (day 0). Etomoxir (30 mg/kg, i.p.) was administered daily starting from day 3. Tumor growth (D) and animal survival (E) were followed. (F) Melanoma-bearing mice received Pmel T cells when tumor sizes have reached 8 to 10 mm in diameters. Etomoxir treatment was initiated 2 d before T cell therapy and given daily for a total of seven doses. Tumors were collected 4 d after T cell therapy for TUNEL assays. Intracellular staining and flow cytometry analyses were performed to evaluate the activation of adoptively transferred Pmel T cells (G) or endogenous CD8+ T cells (H) in the tumors. Data are representative of three independent experiments. *P < 0.05. **P < 0.01. ****P < 0.0001.
