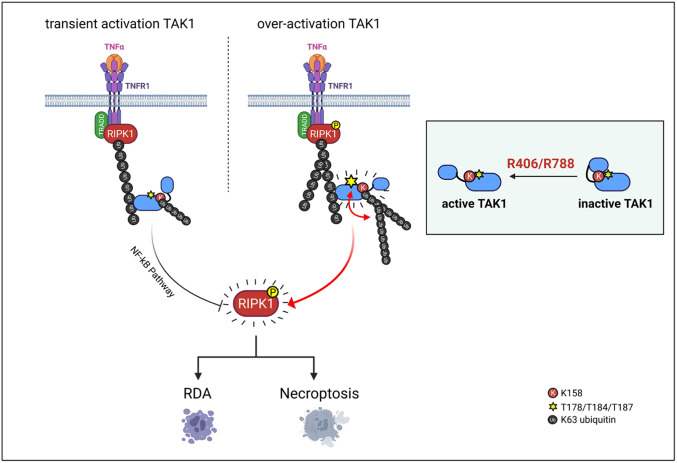
Fig. 8.
A schematic model of R406/R788 promotes TAK1 activity-dependent ubiquitination and TNFα-mediated cell death. Activated TAK1 in R406/R788-stimulated cells preferentially binds with ubiquitinated RIPK1 in complex I within 15 min of stimulation by TNFα. Binding of activated TAK1 with ubiquitinated RIPK1 in R406/R788 treated cells may have three major consequences. The first is the ubiquitination of TAK1 at K158 residue and other sites, which may be mediated by one or more E3 ubiquitinating enzymes that are recruited into complex I that can mediate RIPK1 ubiquitination. The second is the stabilization of activated TAK1 by its ubiquitination. Treatment with R406/R788 leads to sustained activation of TAK1 upto an hour. The third is the activation of RIPK1 mediated by TAK1 phosphorylation. Activated RIPK1 in turn mediates apoptosis and necroptosis.