Figure 2.
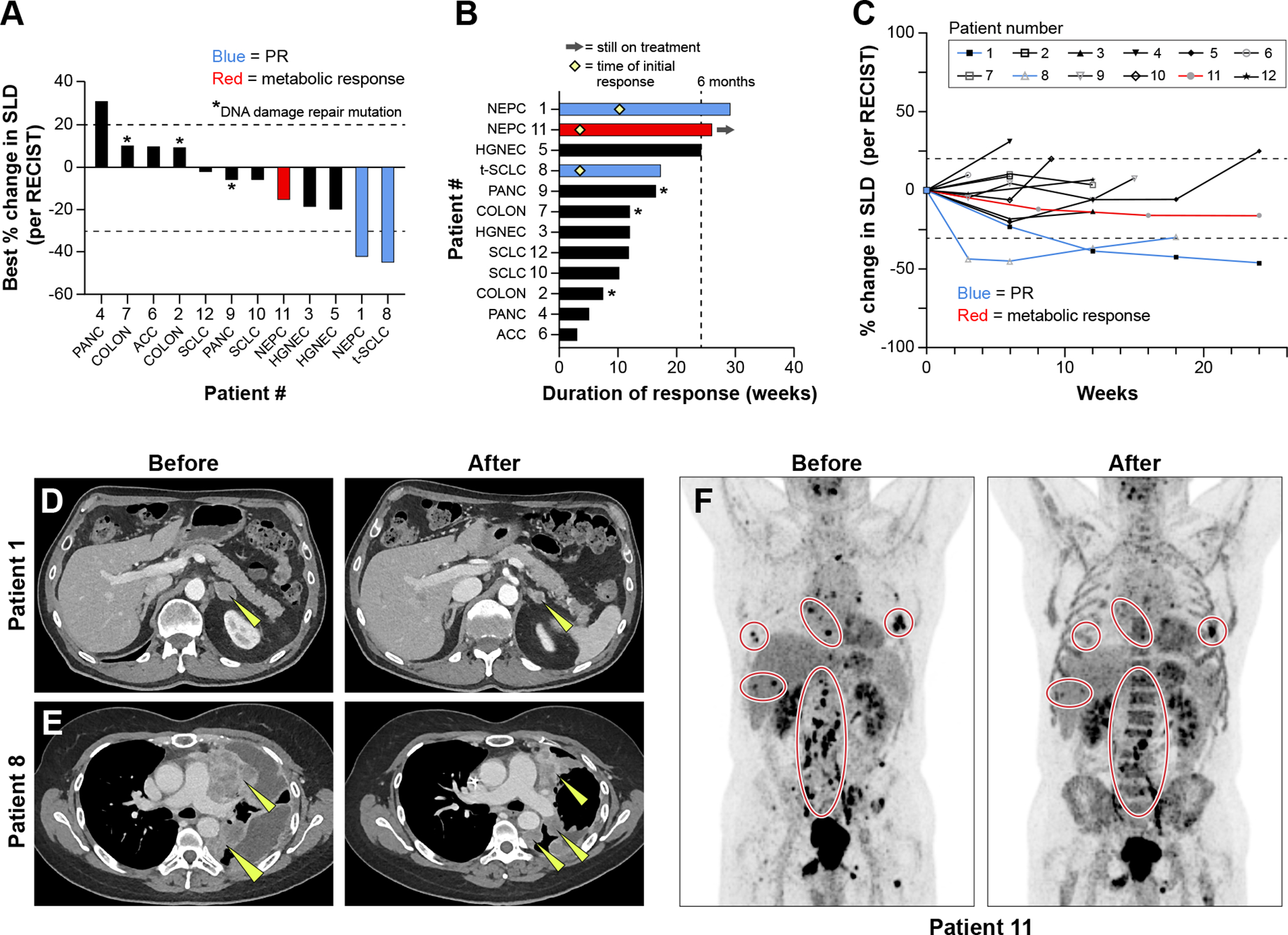
Efficacy of sacituzumab govitecan and berzosertib combination. A: Tumor responses based on maximum change in tumor dimensions from baseline. Each bar represents a patient’s tumor response, corresponding with the assigned patient number. Patients who experienced a partial response per RECIST criteria are annotated in blue, and those with a metabolic response on PET imaging are annotated in red. B: Efficacy based on duration of response, including timing of partial responses, indicated with a yellow diamond, when applicable. C: Efficacy based on change in tumor dimensions from baseline over time. D. Patient 1 with NEPC experienced a partial response, with yellow arrows annotating target lesions. E: Patient 8 with SCLC transformed from EGFR-mutated NSCLC experienced a partial response with a decrease in size of multiple lung masses, as indicated by yellow arrows. F: Patient 11 with de novo NEPC had a metabolic response to therapy across many metastatic sites as shown on PET imaging.
SLD: sum of the total diameter of target lesions
PR: Partial Response per RECIST criteria
PANC = pancreatic adenocarcinoma, COLON = colon adenocarcinoma, ACC = adrenocortical carcinoma, SCLC = small cell lung cancer, NSCLC = non-small cell lung cancer, t-SCLC = SCLC transformed from NSCLC, NEPC = neuroendocrine prostate cancer, HGNEC = high grade neuroendocrine carcinoma
