Figure 6: Absolute platelet counts and platelet activation markers measured throughout the experiment.
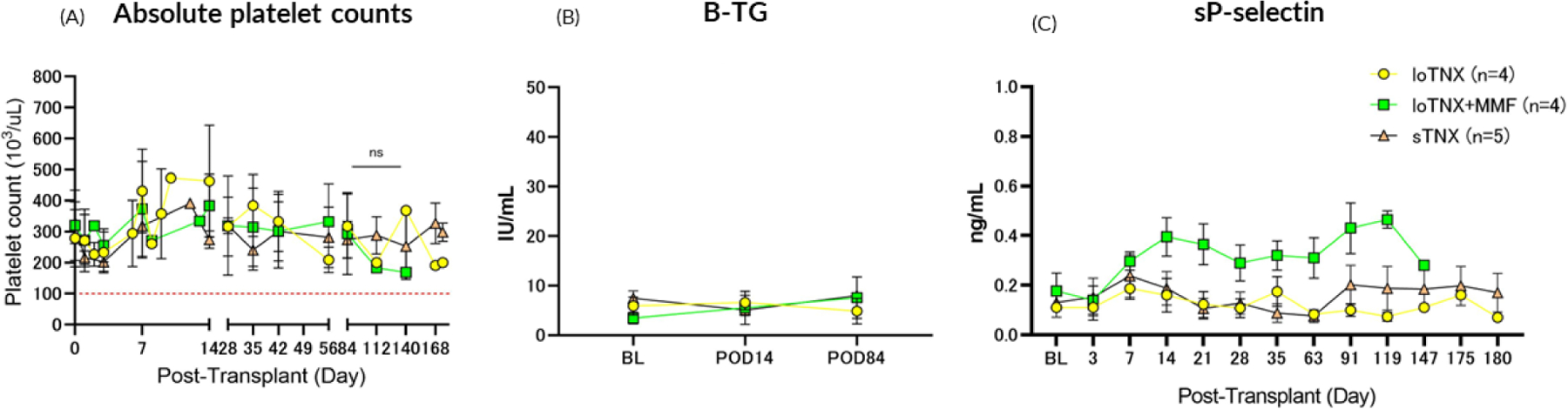
Animals in all treatment groups generally maintained normal platelet counts (above 100 ×103/uL; Fig 6A). Neither sustained thrombocytopenia nor micro- or macrovascular thrombosis were observed during TNX-1500 treatment. Platelet activation, as soluble CD62P and βTG in plasma, remained similar to baseline levels before transplant (BL) in association with either loTNX or stTNX monotherapy (Fig 6B). There was no consistent or statistically significant difference between the TNX monotherapy groups in platelet activation kinetics or in platelet counts throughout treatment relative to baseline BL. The significant sustained elevation in soluble P-selectin in the loTNX+MMF group by the second week and thereafter was unexpected and without obvious explanation.
TNX, TNX-1500; MMF, mycophenolate mofetil
