Abstract
Background:
Efforts to minimize medication risks among older adults include avoidance of potentially inappropriate medications (PIMs). However, most PIMs research has focussed on older people in aged or inpatient care, creating an evidence gap for community-dwelling older adults. To address this gap we investigated the impact of PIMs use in the ASPREE clinical trial cohort.
Methods:
Analysis included 19,114 community-dwelling ASPREE participants aged 70+ years (65+ if US minorities) without major cardiovascular disease, cognitive impairment, or significant physical disability. PIMs was defined according to a modified 2019 AGS Beers Criteria. Cox proportional-hazards regression models were used to estimate the association between baseline PIMs exposure, and disability-free survival, death, incident dementia, disability, and hospitalization, with adjustment for sex, age, country, years of education, frailty, average gait speed, and comorbidities.
Results:
At baseline, 7396 (39% of total) participants were prescribed at least one PIM. Compared with those unexposed, participants on a PIM at baseline were at an increased risk of persistent physical disability (Adjusted HR 1.47, 95%CI 1.21, 1.80) and hospitalization (Adjusted HR 1.26, 95%CI 1.20, 1.32), but had similar rates of disability free survival (Adjusted HR 1.02; 95%CI 0.93, 1.13) and death (Adjusted HR 0.92, 95%CI 0.81, 1.05). These effects did not vary by polypharmacy status in interaction analyses. PIMs exposure was associated with higher risk of disability followed by hospitalization (Adjusted HR 1.92, 95%CI 1.25, 2.96) as well as vice versa (Adjusted HR 1.54, 95%CI 1.15, 2.05). PPIs, anti-psychotics and benzodiazepines were associated with increased risk of disability.
Conclusions:
PIMs exposure is associated with subsequent increased risk of both incident disability and hospitalization. Increased risk of disability prior to hospitalization suggests that PIMs use may start the disability cascade in health older adults. Our findings emphasize the importance of caution when prescribing PIMs in older adults in otherwise good health.
Keywords: 2019 AGS Beers Criteria, Disablement Process, Drug Related, Hospital Related
INTRODUCTION
Medications play a vital role in primary prevention and health maintenance, but any use of medication requires careful consideration of the balance between risk and benefit. This balance is particularly important in older adults, in whom physiological changes associated with aging alter the pharmacokinetic and pharmacodynamic response to medications leading to increased vulnerability to their adverse effects (1,2). Medications shown to be associated with excess morbidity (relative to potential benefit) in older adults are identified as ‘potentially inappropriate medications’ (PIMs) (3). Several explicit criteria, including the AGS Beers Criteria® and the STOPP criteria, have been developed to identify PIMs and guide prescribing decisions in older adults. PIMs have been associated with increased risk of hospitalization, worsening of physical function and death in vulnerable populations, such as those with cognitive impairment, or in aged care and inpatient care (4-8).
This research focus on PIMs in vulnerable populations creates an evidence-gap. Life expectancy at older ages is increasing (9) and, at the same time, the proportion of older people reporting fair or poor health status and significant functional limitations is decreasing (10). Many individuals now reach older age in relatively good health and live in the community without the confounding underlying morbidity and fraility that are prevalent in the vulernable populations where PIMs risks have been established. We have previously reported that community-dwelling ‘healthy’ older people have similar prevalence of PIMs to the general older population (11) but it is not known whether these medications carry increased risk of harm for healthy older adults as has been reported in those with poorer health status. Furthermore, previous research into PIMs has focussed on definitive outcomes such as mortality or hospitalization but not functional outcomes such as the ability to perform daily tasks, which are key to ongoing independence. Understanding the risk profile of PIMs in healthy older adults is necessary to determine how to balance the potential risks of certain medications against the potential benefits in this growing population group.
ASPirin in Reducing Events in the Elderly (ASPREE) was a randomized, placebo-controlled primary prevention trial of 100mg daily aspirin in community-dwelling older adults in Australia and the US (12). Participants were required to be in good health, free of pre-existing major cardiovascular disease, cognitively intact and able to independently perform basic activities of daily living. In this analysis, we aimed to determine if baseline PIMs use in community-dwelling older adults was a) associated with poorer functional outcomes (disability free survival, death, incident dementia, incident persistent disability) and hospitalization, and b) whether any risks observed were attributable to specific classes of PIMs.
METHODS
ASPREE Clinical Trial
This is a secondary analysis of data from ASPREE. Briefly, 19,114 healthy people aged 70 years or older (65 or older for US minorities) were randomized in Australia (n=16,703) and the US (n=2,411) (12). Recruitment commenced March 2010 and the trial concluded in June 2017, with a median 4.7 years of follow-up involving annual in-person study visits conducted between 2011 and 2017. At baseline, participants had lower prevalence of diabetes mellitus, osteoarthritis, chronic kidney disease, obesity, dyslipidemia and smoking and hence, were generally healthier than the broader population of a similar age (12). Furthermore, participants reported higher health-related quality of life (HRQoL) than the general older population (13). Detailed methods and results of ASPREE are described elsewhere (12,14).
Collection of medication from participants
Participants were asked to bring their medications, or a current medication list, to their baseline data collection visit. Research staff reviewed each medication and confirmed whether the medication was prescribed by the participant’s doctor. All prescription medications were coded according to the World Health Organisation Anatomical and Therapeutic Chemical (ATC) coding system (https://whocc.no/atc_ddd_index/). Detailed methods of the medication collection and coding process have been described elsewhere (15).
Definitions
Potentially inappropriate medications (PIMs)
PIMs were defined as any medication where the overall risk associated with their use may outweigh possible benefit. For this analysis, any medication listed under Table 2 of the 2019 AGS Beers Criteria® for PIM use in older adults was included (3). Rather than using all the medications from the criteria, this subgroup of medications was chosen because of the strong recommendation to avoid, as opposed to other subgroups of medications that should be used with caution or avoided only for certain disease states. Where the data necessary to determine if the medication met the AGS Beers criteria® (e.g., lack of dose, dosing regimen or indication data) were not collected, the medication was not considered to be PIM (e.g., insulin sliding scale). Proton pump inhibitors (PPIs) were considered PIMs if they were not co-prescribed with a Non-steroidal Anti-Inflammatory Drug (NSAID). This analysis pertains to baseline medications that were prescribed prior to the commencement of study medication (i.e. aspirin or placebo) for the trial. Further rationale for the choice of this criteria has been published elsewhere (11), and a full list of PIMs used in this analysis and medications that were excluded from PIMs analysis is included in Supplementary Table S1.
Table 2 –
Risk of disability free survival, death, dementia, persistent disability or hospitalization outcomes by baseline PIMs exposure, based on modified 2019 AGS Beers Criteria®
| No PIM (n=11,718) |
PIM (n=7,396) |
Unadjusted hazard ratio (95% CI) |
Adjusted hazard ratio (95% CI)a |
|||
|---|---|---|---|---|---|---|
| N | Rate per 1000 person years (95% CI) |
N | Rate per 1000 person years (95% CI) |
|||
| Loss of disability free survival b | 1086 | 20.5 (19.3, 21.7) | 749 | 22.7 (21.1, 24.4) | 1.12 (1.02, 1.23) | 1.03 (0.94, 1.13) |
| Death | 655 | 12.0 (11.2, 13.0) | 397 | 11.7 (10.6, 12.9) | 0.98 (0.86, 1.11) | 0.92 (0.81, 1.05) |
| Incidentc disability | 197 | 4.1 (3.6, 4.8) | 215 | 7.3 (6.4, 8.4) | 1.8 (1.50, 2.20) | 1.47 (1.21, 1.80) |
| Incident dementiac | 357 | 6.9 (6.2, 7.6) | 218 | 6.7 (5.9, 7.7) | 0.98 (0.83, 1.17) | 0.93 (0.79, 1.11) |
| Incident Hospitalization | 4196 | 95.5 (92.6, 98.4) | 3245 | 126.3 (122.0, 130.1) | 1.33 (1.20, 1.40) | 1.26 (1.20, 1.32) |
Adjusted for sex, age, country, years of education (<12y, vs 12y+), frailty, average gait speed, hypertension, diabetes, and the presence of depression.
Composite of first occurance of death from any cause, incident dementia or incident physical disability
Incident includes the first occurance of disability or dementia regardless of whether the event was preceded by another event of interest (e.g. incident disability includes the first occurrence of disability regardless of whether that disability was preceded by dementia)
Covariates
Hypertension was defined as systolic blood pressure of ≥140 mmHg or diastolic blood pressure ≥ 90 mmHg or pharmaceutical treatment for high blood pressure. Diabetes was defined as self-report of diabetes or fasting blood glucose of ≥126 mg/dL or on pharmaceutical treatment for diabetes. Frailty was categorized on the basis of adapted Fried frailty criteria, which included body weight, strength, exhaustion, walking speed, and physical activity (16). The category of prefrail included participants who met one or two of these five criteria, and the category of frail included those who met three or more criteria. Polypharmacy was defined as presence of 5 or more prescription medications.
Outcomes
Loss of disability-free survival (hereafter DFS) was assessed using a composite of the first occurrence of death from any cause, dementia, or persistent physical disability. Persistent physical disability (hereafter disability) was defined as experiencing ‘a lot of difficulty’ or requiring assistance for any one of six basic activities of daily living for a period of at least 6 months (14). Dementia was confirmed using DSM-IV criteria by an independent adjudication committee. Details of the adjudication process have been published elsewhere (14). Incident disability and incident dementia analysis included the first occurrence of the event of interest regardless of whether it was preceded or followed by another event of interest. We also examined the association between PIMs and hospitalizations. In this analysis, incident hospitalization was defined as first admission to hospital for a period of 24 hours or more, for any reason.
Statistical analysis
Descriptive statistics (frequencies, percentages, means) were used to summarize PIMs’ prevalence data. Unadjusted odds ratios (OR) were used to describe associations between baseline PIMs’ exposure and each of age, study drug treatment group, polypharmacy and physical function (the ability to walk up one flight of stairs). Cox proportional-hazards regression models were used to estimate the association between baseline exposure to PIMs and DFS, and each of death, incident dementia, disability, and hospitalization. Models were then adjusted for previously identified confounders of PIMs within the ASPREE cohort (11): sex, age, country, years of education, frailty, average gait speed (usual walking speed over 3 meters), hypertension, diabetes, polypharmacy and the presence of depression (using the Center for Epidemiological Studies Depression (CESD)-10 questions score of ≥ 8) (12) at baseline. Confidence intervals were not adjusted for multiple comparisons. To disentangle the impact of PIMs from polypharmacy, a test for interaction between polypharmacy and PIM was conducted, and an analysis stratified by baseline polypharmacy is presented. To explore the relationship between disability and hospitalization we conducted sensitivity analysis in those who experienced both events.
RESULTS
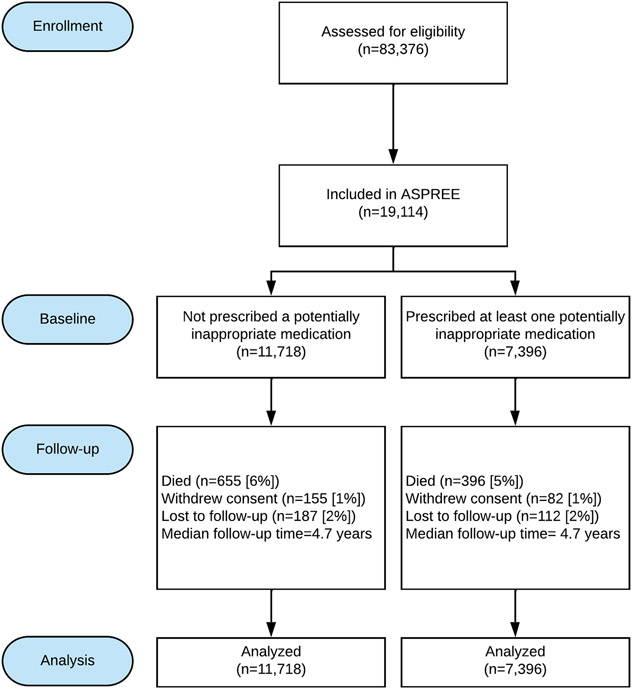
At baseline, 7396 (39% of total) participants had prescription for at least one PIM (Figure 1). The proportion of participants who became lost to follow-up or died was similar between those with, and without a PIM, as was median follow-up time. Of those prescribed a PIM at baseline, 79% remained on at least one PIM throughout follow-up. Of those who were not prescribed a PIM at baseline, 46% were prescribed a PIM at some stage during follow-up. Participants who were aged 65-69 years were less likely (OR 0.72; 95%CI 0.59, 0.86) to be prescribed a PIM compared with those aged 70-74 and those aged 80-84 were more likely to be on a PIM (OR 1.22; 95%CI 1.11, 1.34), as were participants with polypharmacy or who reported any difficulty walking up a flight of stairs (Table 1). PPIs prescribed without concurrent NSAID use (54.8% of participants with PIMs use) were the most common PIMs, followed by NSAIDs without concurrent PPI use (17.3%), benzodiazepines (17%), androgens and estrogens (11.8%), and drugs with anti-cholinergic properties (11.6%) (see Table S2). With regard to PPI use, 4714 participants were prescribed PPIs at baseline(17). Of these 4054 (86%) were considered PIMs based on the 2019 AGS Beers Criteria®.
Figure 1: Participant follow-up by baseline PIMs exposure.
For participants who withdrew from the trial, all information up to the point of withdrawal was included in the analysis.
Table 1 –
Participant baseline characteristics grouped by presence or absence of PIMs, based on modified 2019 AGS Beers Criteria®
| Group | No PIM (n=11,718) |
PIM (n=7,396) |
Odds Ratio (95% CI) |
|---|---|---|---|
| Age (n and percent) | |||
| 65-69 | 393 (3%) | 173 (2%) | 0.72 (0.59,0.86) |
| 70-74 | 6,576 (56%) | 4,022 (54%) | Reference |
| 75-79 | 3,045 (26%) | 1,977 (27%) | 1.06 (0.99,1.14) |
| 80-84 | 1,098 (9%) | 840 (11%) | 1.22 (1.11,1.34) |
| 85+ | 606 (5%) | 384 (5%) | 1.04 (0.89,1.22) |
| Treatment group (n and percent) | |||
| Placebo | 5,817 (50%) | 3,708 (50%) | Ref |
| Aspirin | 5,901 (50%) | 3,688 (50%) | 1.02 (0.96,1.08) |
| Polypharmacy a | |||
| No polypharmacy | 9,885 (84%) | 4,141 (56%) | Ref |
| Polypharmacy | 1,833 (16%) | 3,255 (44%) | 4.24 (3.94,4.55) |
| Difficulty walking up one flight of stairs | |||
| No difficulty | 10,295 (88%) | 5,970 (81%) | Ref |
| Any level of difficulty | 1,423 (12%) | 1,426 (19%) | 1.72 (1.59, 1.87) |
Polypharmacy defined as concurrent use of 5 or more prescription medications
As shown in Table 2, comparing those without exposure to PIM to participants on a PIM, on adjusted analyses there was no clear evidence of a difference in risk of the loss of DFS (Adjusted HR 1.03; 95%CI 0.94, 1.13), death (Adjusted HR 0.92, 95%CI 0.81, 1.05) or incident dementia (Adjusted HR 0.93, 95%CI 0.79, 1.11). However, participants on a PIM had a higher rate of disability (7.3 per 1000 person years PIM vs 4.1 No PIM; Adjusted HR 1.47, 95%CI 1.21, 1.80) and hospitalization for any reason (126.3 per 1000 person years PIM vs 95.5 No PIM; Adjusted HR 1.26, 95%CI 1.20, 1.32). Of those who developed disability, 48% had previously reached the hospitalization outcome (n=198), 22% reached the hospitalization outcome after developing disability (n=92) and 30% were never hospitalized. For those with a prior hospitalization, the median time between hospitalization and disability was 401 days (IQR 181-857 days). For those with a subsequent hospitalization, the median time between disability and hospitalization was 247 days (IQR 73.5 – 469 days). Additional analysis of the disability and hospitalization outcomes is shown in Table S3. Those with exposure to PIM had higher risk of both hospitalization followed by disability (Adjusted HR 1.54, 95%CI 1.15, 2.05), and disability followed by hospitalization (Adjusted HR 1.92, 95%CI 1.25, 2.96).
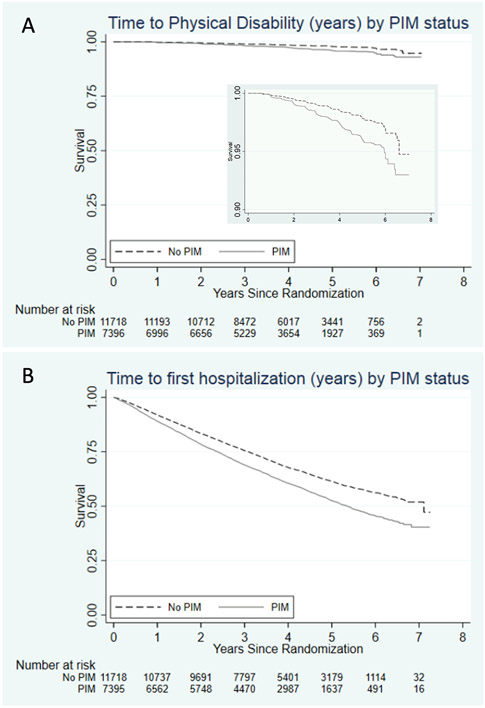
Table 3 shows relationships between PIMs and outcomes stratified by polypharmacy. In those without polypharmacy, PIM exposure groups had similar risk for loss of DFS (Adjusted HR 0.95, 95% CI 0.83, 1.08), death (Adjusted HR 0.87, 95%CI 0.73, 1.03) and dementia (Adjusted HR 0.86, 95%CI 0.70, 1.08), but for the other outcomes participants on a PIM had a higher rates of disability (5.1 per 1000 person years PIM vs 3.4 No PIM; Adjusted HR 1.41, 95%CI 1.07, 1.86) and hospitalization (114.2 per 1000 person years PIM vs 89.2 per No PIM; Adjusted HR 1.23, 95%CI 1.16, 1.31) (Table 2 and Figure 2). However, in those with polypharmacy, similar rates of loss of DFS (Adjusted HR 0.98, 95%CI 0.84, 1.15), death (Adjusted HR 0.85, 95%CI 0.69, 1.06), disability (Adjusted HR 1.16, 95%CI 0.84, 1.57), dementia (Adjusted HR 1.07, 95%CI 0.78, 1.46) and incident hospitalization (Adjusted HR 1.04, 95%CI 0.95, 1.13) were observed between PIM exposure groups.
Table 3 –
Risk of disability free survival, death, dementia, disability and hospitalization outcomes by baseline PIMs exposure, based on modified 2019 AGS Beers Criteria®, stratified by polypharmacy status
| No PIM (n=11,718) |
PIM (n=7,396) |
Adjusteda Hazard Ratio |
||||||
|---|---|---|---|---|---|---|---|---|
| N | Rate per 1000 person years (95% CI) |
N | Rate per 1000 person years (95% CI) |
HR (95%CI) | ||||
| No polypharmacy (n = 14,026 ) | ||||||||
| Loss of disability free survival b | 846 | 18.8 (17.6, 20.2) | 341 | 18.2 (16.3, 20.2) | 0.95 (0.83, 1.08) | |||
| Death | 509 | 11.1 (10.1, 12.1) | 188 | 9.8 (8.5, 11.3) | 0.87 (0.73, 1.03) | |||
| Incident disabilityc | 137 | 3.4 (2.9, 4.0) | 86 | 5.1 (4.2, 6.4) | 1.41 (1.07, 1.86) | |||
| Incident dementiac | 297 | 6.8 (6.0, 7.6) | 109 | 5.9 (4.9, 7.4) | 0.86 (0.70, 1.08) | |||
| Incident Hospitalization | 3368 | 89.2 (86.3, 92.3) | 1699 | 114.2 (108.9, 119.7) | 1.23 (1.16, 1.31) | |||
| Polypharmacy (n= 5088) | ||||||||
| Loss of disability free survival b | 240 | 29.7 (26.2, 33.7) | 408 | 28.7 (25.9, 31.6) | 0.98 (0.84, 1.15) | |||
| Death | 146 | 17.4 (14.8, 20.5) | 209 | 14.1 (12.3, 16.2) | 0.85 (0.69, 1.06) | |||
| Incident disabilityc | 60 | 8.4 (6.5, 10.8) | 129 | 10.3 (8.7, 12.2) | 1.16 (0.84, 1.57) | |||
| Incident dementiac | 60 | 7.6 (5.9, 9.8) | 109 | 7.8 (6.5, 9.4) | 1.07 (0.78, 1.46) | |||
| Incident Hospitalization | 828 | 133.5 (124.8, 143.0) | 1546 | 143.0 (136.0, 150.3) | 1.04 (0.95, 1.13) | |||
Adjusted for sex, age, country, years of education (<12y, vs 12y+), frailty, average gait speed, hypertension, diabetes, and the presence of depression.
Composite endpoints of death from any cause, incident dementia or incident physical disability.
Incident includes the first occurance of disability or dementia regardless of whether the event was preceded by another event of interest (e.g. incident disability includes the first occurrence of disability regardless of whether that disability was preceded by dementia).
Figure 2: Kaplan-Meier curves for Disability and Hospitalization by baseline PIMs status (inset panel on figure A is the same curve with modified y-axis range of 0.90 - 1.00).
Supplementary Table S4 reports hazard ratios for comparison of PIM between those with and without polypharmacy, including an interaction between PIM and polypharmacy. The interaction term is significant only for hospitalization, and the relevant combination of coefficients suggests that compared to those with PIM and not polypharmacy, those with PIM and polypharmacy do not exhibit elevated risk, all other variables held constant.
Table S5 shows the relationship between the individual classes of PIM and clinical outcomes. There was an increased risk of disability associated with use of antipsychotics (Adjusted HR 1.96, 95%CI 1.17, 3.28), PPIs (Adjusted HR 1.34, 95%CI 1.07, 1.67), and benzodiazepines (Adjusted HR 1.38, 95%CI 1.01, 1.89). PPI use that met the criteria for PIM was associated with a lower risk of death following adjustment for confounders (12.1 events for 1000 person years No PIM vs 11.0 PIM, Adjusted HR 0.85, 95%CI 0.73, 0.99) and Table S6 shows cause of death within this group. No difference in rates was detected for any of the outcomes for cardiovascular PIMs or pain medications excluding NSAIDs.
DISCUSSION
Prescribing medications that have been identified as potentially inappropriate for older people requires consideration of the individual circumstances. In clinical practice, it may be deemed that the benefits outweigh the risks for a given individual, especially for adults who have reached older ages free of significant life-limiting illness or disability and therefore may be more robust. However, after adjustment for baseline comorbidities including frailty, we have found that PIM exposure in healthy older adults without major cardiovascular disease or baseline disability is associated with higher rates of incident physical disability and hospitalization. We found no difference in risk for death or dementia, both of which occurred more commonly than disability, and this resulted in a null finding for the broader category of disability free survivial.
Potential clinical impact of findings related to PIMs and physical disability
To our knowledge, this is the first study to explicitly explore associations between PIMs exposure and disability (defined as the persistent loss of at least one ADL) in community-dwelling older adults. Although there is a well-established association between specific PIMs and fracture (18-20), and fractures have been associated with disability (21), previous studies have lacked the longitudinal data required to explore disability based on persistent loss of functionality associated with activites of daily living. Recent studies of more vulnerable older people have shown that PIMs are associated with increased risk of functional decline following hospitalization (22), which suggests that hospitalization and disability may be components on a disease continuum with a hospitalization for PIMs initiating a cascade of events that results in functional decline. This raises the question as to whether hospitalization, which is a well established risk associated with PIMs use, may be driving the increased risk we observed for disability. However, this does not appear to be the case. Almost half of those who developed disability did not have a prior hospitalization. Sensitivity analysis found that PIMs exposure was associated with an increased risk of disability specifically in those for whom disability preceded hospitalization. This suggests that PIMs may increase the risk of disability through pathways that are not linked with prior hospitalizations, and may even initiate the disability cascade in healthy older adults. Persistent loss of an ADL in older people has been associated with substantial reduction in health related quality of life (23). The ability to live independently is a critical factor in aging and loss of independence is a known fear among the general older population (24). Bearing this in mind, our findings of an almost 50% increase in the rate of persistent physical disability associated with exposure to PIMs suggests that caution is warranted when prescribing PIMs to older adults regardless of their health and function.
Context and interpretation of hospitalization and death findings
Findings of increased risk of hospitalization associated with PIMs exposure in our study were similar to those previously published (25-27), adding strength to the evidence that PIM use is associated with increased hospitalization not only in more vulnerable populations but also healthier older adults.
Overall, we did not observe any difference between the risk of death in those with PIM exposure compared to those without PIMs. While this result is consistent with recent studies in modestly sized cohorts (~500-600 people) of older people discharged from hospital or residents in nursing home who were likely to be more unwell (28,29), it contrasts with results of a larger (n=1606) study in Brazil that showed a 44% increase in the risk of death for PIMs using the 2012 AGS Beers Criteria® (30). Our study utilized the updated 2019 AGS Beers Criteria® and therefore included PPIs, which may explain the differences in results as PPIs were associated with a decreased risk of death on our cohort (see further discussion below). Notably, two recent studies have shown an association between increased risk of death and new initiation of PIMs but not longer term use (31,32). If ASPREE participants represent longer term PIMs users, then our findings would be in line with these two studies. However, ASPREE did not collect data on historical medication use and hence it was not possible to determine when those on PIMs at baseline were initially prescribed them.
Impact of polypharmacy on PIMs findings
In analysis stratified by polypharmacy, the raw rates of disability and hospitalization were higher in those with polypharmacy compared to those without polypharmacy regardless of PIM status. This is consistent with the well known risks of polypharmacy as an independent predictor of hospitalization and death in older people (33). However, we found that participants without polypharmacy who were exposed to PIMs had an increased risk of disability or hospitalization. This suggests that PIMs should still be prescribed but with caution for older people without polypharmacy.
Risk profile of individual PIMs
No single PIMs class appeared to be responsible for the increased risks we observed, and anti-psychotics, benzodiazepines and PPIs were all independently associated with increased risk of disability in our cohort. Given their established adverse effects, both antipsychotics and benzodiazepines have been consistently included on the list of PIMs from the initial publication by Beers (34) until the most recently released AGS Beers criteria (in 2019) (3). However, PPIs were only recently included. PPI use without appropriate clinical indication or concurrent NSAID use was added to the 2019 criteria based on evidence of increased risk of Clostridium difficile infection and bone loss and fractures (3). Given that fractures are a risk factor for disability (35,36), our findings of increased risk of disability with PPI use is consistent with this rationale. However, we also observed association between PPI use and decreased risk of death. This beneficial association is in conflict with previous studies conducted in the general population and in more vulnerable older populations, where PPIs have been linked with increased risk of both mortality (37,38). The raw difference in mortality rates between those exposed to PPIs that met the criteria and those not exposed was small, and therefore it is possible that our mortality finding is an erroneous result that is attributable to adjustment for confounders. Additionally, our analysis was limited to medications that met the PIMs criteria according to Table 2 of the 2019 AGS Beers Criteria®. This meant that not all PPIs were considered to be PIMs, and examination of broader PPI use was beyond the scope of our analysis. While efforts have been made in recent years to reduce the number of older adults on high dose PPIs, longer term use of high-dose PPIs remains common in Australia, US, France and Iceland (39-43) . Therefore, we believe further investigation of the associations between PPI use and functional outcomes, and analysis of PPI use more broadly, may be warranted given the substantial proportion of older people taking this class of medication.
Potential impact of changes in prescription medication use during follow-up
Older people may change medications frequently. We chose to look at the impact of PIMs exposure at a certain point in time (baseline in the ASPREE study) rather than ongoing or time varying exposure in order to explore the simple clinical question of whether an older person who is currently on a PIM (for any reason) is at increased risk of poor functional outcomes. While the majority of participants (79%) who were on a PIM at baseline remained on a PIM throughout the entire follow-up period, many of those not prescribed PIMs at baseline commenced on a PIM at some point during follow-up (46%). If anything, initiation of PIMs in the No PIM group likely diluted the true difference between the the groups and result in an underestimation of the risk of disability associated with PIMs use. Therefore, we do not believe our findings are undermined by our chosen methodology, but instead reflect the real world prescribing environment.
Strengths & Limitations
A key strength of our study is the prospective design with regular physical disability screening and robust clinical event adjudication, which minimized ascertainment bias. We used a validated PIMs tool. We used a large sample of healthy older adults and were able to control for a wide range of demographic, lifestyle and known risk factors. Given the observational design, it is not possible to evaluate causality. Reverse causality is a major factor in measuring the association between medications and health outcomes. However, our participants were clinically free of dementia, myocardial infarction, stroke and other cardiovascular disease, such as transient ischemic attack or angina, at baseline and were independent with all ADLs. Overall, data quality was high with limited missing data (44).
This analysis accounted for a wide range of potential confounding variables, but potential for residual confounding remains. Our analysis was based on the presence of PIMs at study entry, and we did not collect medication dose, nor did we collect non-prescription medications other than NSAID use. Length of exposure prior to randomization was not collected, and therefore we cannot rule out a selection bias caused by an impact of PIMs prior to enrollment. We could not account for changes in PIM exposure during follow-up. There was also a lack of information regarding the clinical indication surrounding the PIM, which limits the detail to which the 2019 AGS Beers Criteria® could be definitively applied. Previous research has shown that use of explicit criteria to evaluate prescribing may account for only a small portion of drugs deemed inappropriate by implicit review (45). Ascertainment of medications relied on self-report and the checking of packaging and prescriptions by study staff. However, wherever possible, medical records were used to prompt participants about medications they have may omitted, mitigating the limitations of self-report.
CONCLUSION
PIMs exposure at baseline was associated with increased the rates of incident physical disability and/or incident hospitalization in healthy older people without significant baseline cardiovascular, cognitive or physical impairment. Our findings emphasize the importance of caution when prescribing PIMs in older adults, including those in otherwise good health.
Supplementary Material
Table S1: Medications included and excluded as PIMs for this analysis based on modified AGS Beers Criteria.
Table S2: Baseline PIMs prevalence by medication class.
Table S3: Risk of disability and hospitalization outcomes by baseline PIMs exposure, in participants who experienced both events.
Table S4: Assessment of interaction between Cox models for PIMs and Polypharmacy.
Table S5: Risk of primary endpoint, death, dementia, disability and hospitalisation outcomes by baseline PIMs medication category exposure.
Table S6: Risk of cause of death from specific causes by baseline PPI use.
Key Points
Among community-dwelling healthy older adults, PIMs use was associated with significantly increased risk of incident disability (defined as persistent loss of at least one ADL), and hospitalization.
Increased risk of disability with PIMs use was evident in those for whom disability preceeded hospitalization, as well as vice versa.
Polypharmacy did not modify the associations, and PIMs users without concurrent polypharmacy were at increased risk of disability and hospitalization.
What does this matter?
Our findings suggest that PIMs use may start the disability cascade among healthy community-dwelling older adults and confirm that caution is warranted when prescribing PIMs to older adults regardless of their health, function and polypharmacy status.
ACKNOWLEDGEMENTS
A. G. Bayer provided aspirin and matching placebo. The authors acknowledge the dedicated and skilled staff in Australia and the Unites States for the conduct of the trial. The authors also are most grateful to the ASPREE participants, who so willingly volunteered for this study, and the general practitioners and medical clinics who supported the participants in the ASPREE study. Trial Registration: International Standard Randomized Controlled Trial Number Register (ISRCTN83772183) and clinicaltrials.gov (NCT01038583).
Funding:
The work was supported by the National Institute on Aging and the National Cancer Institute at the National Institutes of Health (U01AG029824); the National Health and Medical Research Council (NHMRC) of Australia (334047 and 1127060); Monash University and the Victorian Cancer Agency. JEL is funded through a Fulbright Postdoctoral Fellowship.
Sponsor’s role
Funding sources were not involved in the design or conduct of the study; collection, management, or analysis of the data; interpretation of the results; preparation, review, or approval of the manuscript; or decision to submit for publication.
Footnotes
Conflict of interest
The authors have no conflict of interest to declare in relation to this study.
ASPREE Investigator Group listed on www.aspree.org
Some of the content of this paper was presented at the 2022 AGS conference.
REFERENCES
- 1.Turnheim K. When drug therapy gets old: pharmacokinetics and pharmacodynamics in the elderly. Exp Gerontol. 2003. Aug;38(8):843–53. [DOI] [PubMed] [Google Scholar]
- 2.Oscanoa TJ, Lizaraso F, Carvajal A. Hospital admissions due to adverse drug reactions in the elderly. A meta-analysis. Eur J Clin Pharmacol. 2017. Jun 1;73(6):759–70. [DOI] [PubMed] [Google Scholar]
- 3.American Geriatrics Society 2019 Updated AGS Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2019;67(4):674–94. [DOI] [PubMed] [Google Scholar]
- 4.Lau DT, Kasper JD, Potter DEB, Lyles A, Bennett RG. Hospitalization and Death Associated With Potentially Inappropriate Medication Prescriptions Among Elderly Nursing Home Residents. Arch Intern Med. 2005. Jan 10;165(1):68–74. [DOI] [PubMed] [Google Scholar]
- 5.Dedhiya SD, Hancock E, Craig BA, Doebbeling CC, Thomas J. Incident use and outcomes associated with potentially inappropriate medication use in older adults. Am J Geriatr Pharmacother. 2010. Dec 1;8(6):562–70. [DOI] [PubMed] [Google Scholar]
- 6.Lin HY, Liao CC, Cheng SH, Wang PC, Hsueh YS. Association of Potentially Inappropriate Medication Use with Adverse Outcomes in Ambulatory Elderly Patients with Chronic Diseases: Experience in a Taiwanese Medical Setting. Drugs Aging. 2008;25(1):49–59. [DOI] [PubMed] [Google Scholar]
- 7.Chin MH, Wang LC, Jin L, Mulliken R, Walter J, Hayley DC, et al. Appropriateness of Medication Selection for Older Persons in an Urban Academic Emergency Department. Acad Emerg Med. 1999;6(12):1232–41. [DOI] [PubMed] [Google Scholar]
- 8.Cross AJ, George J, Woodward MC, Ames D, Brodaty H, Wolfe R, et al. Potentially Inappropriate Medication, Anticholinergic Burden, and Mortality in People Attending Memory Clinics. J Alzheimers Dis JAD. 2017;60(2):349–58. [DOI] [PubMed] [Google Scholar]
- 9.Statistics (US) NC for H. Table 14, Life expectancy at birth and at age 65, by sex: Organisation for Economic Co-operation and Development (OECD) countries, selected years 1980–2015 [Internet]. 2018. [cited 2019 Dec 22]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK532684/table/ch4.tab14/
- 10.Statistics (US) NC for H. Trend Tables [Internet]. National Center for Health Statistics (US); 2018. [cited 2019 Dec 22]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK532684/ [Google Scholar]
- 11.Lockery JE, Ernst ME, Broder JC, Orchard SG, Murray A, Nelson MR, et al. Prescription Medication Use in Older Adults Without Major Cardiovascular Disease Enrolled in the Aspirin in Reducing Events in the Elderly (ASPREE) Clinical Trial. Pharmacother J Hum Pharmacol Drug Ther. 2020;40(10):1042–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McNeil JJ, Woods RL, Nelson MR, Murray AM, Reid CM, Kirpach B, et al. Baseline Characteristics of Participants in the ASPREE (ASPirin in Reducing Events in the Elderly) Study. J Gerontol A Biol Sci Med Sci. 2017. Oct;72(11):1586–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stocks NP, González-Chica DA, Woods RL, Lockery JE, Wolfe RSJ, Murray AM, et al. Quality of Life for 19,114 participants in the ASPREE (ASPirin in Reducing Events in the Elderly) study and their association with sociodemographic and modifiable lifestyle risk factors. Qual Life Res Int J Qual Life Asp Treat Care Rehabil. 2019. Apr;28(4):935–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McNeil JJ, Woods RL, Nelson MR, Reid CM, Kirpach B, Wolfe R, et al. Effect of Aspirin on Disability-free Survival in the Healthy Elderly. N Engl J Med. 2018. Sep 16;0(0):null. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lockery JE, Rigby J, Collyer TA, Stewart AC, Woods RL, McNeil JJ, et al. Optimising medication data collection in a large-scale clinical trial. PLOS ONE. 2019. Dec 27;14(12):e0226868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolfe R, Murray AM, Woods RL, Kirpach B, Gilbertson D, Shah RC, et al. The aspirin in reducing events in the elderly trial: Statistical analysis plan. Int J Stroke Off J Int Stroke Soc. 2018. Apr;13(3):335–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahady SE, Margolis KL, Chan A, Polekhina G, Woods RL, Wolfe R, et al. Major GI bleeding in older persons using aspirin: incidence and risk factors in the ASPREE randomised controlled trial. Gut. 2021. Apr;70(4):717–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thong BKS, Ima-Nirwana S, Chin KY. Proton Pump Inhibitors and Fracture Risk: A Review of Current Evidence and Mechanisms Involved. Int J Environ Res Public Health [Internet]. 2019. May [cited 2019 Dec 18];16(9). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6540255/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madhusoodanan S, Bogunovic OJ. Safety of benzodiazepines in the geriatric population. Expert Opin Drug Saf. 2004. Sep;3(5):485–93. [DOI] [PubMed] [Google Scholar]
- 20.Glass J, Lanctôt KL, Herrmann N, Sproule BA, Busto UE. Sedative hypnotics in older people with insomnia: meta-analysis of risks and benefits. BMJ. 2005. Nov 19;331(7526):1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bertram M, Norman R, Kemp L, Vos T. Review of the long-term disability associated with hip fractures. Inj Prev. 2011. Dec 1;17(6):365–70. [DOI] [PubMed] [Google Scholar]
- 22.Fabbietti P, Ruggiero C, Sganga F, Fusco S, Mammarella F, Barbini N, et al. Effects of hyperpolypharmacy and potentially inappropriate medications (PIMs) on functional decline in older patients discharged from acute care hospitals. Arch Gerontol Geriatr. 2018. Jul 1;77:158–62. [DOI] [PubMed] [Google Scholar]
- 23.Lyu W, Wolinsky FD. The Onset of ADL Difficulties and Changes in Health-Related Quality of Life. Health Qual Life Outcomes. 2017. Nov 6;15(1):217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quine S, Morrell S. Fear of loss of independence and nursing home admission in older Australians. Health Soc Care Community. 2007. May;15(3):212–20. [DOI] [PubMed] [Google Scholar]
- 25.Lu WH, Wen YW, Chen LK, Hsiao FY. Effect of polypharmacy, potentially inappropriate medications and anticholinergic burden on clinical outcomes: a retrospective cohort study. CMAJ Can Med Assoc J. 2015. Mar 3;187(4):E130–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xing XX, Zhu C, Liang HY, Wang K, Chu YQ, Zhao LB, et al. Associations Between Potentially Inappropriate Medications and Adverse Health Outcomes in the Elderly: A Systematic Review and Meta-analysis. Ann Pharmacother. 2019. Oct 1;53(10):1005–19. [DOI] [PubMed] [Google Scholar]
- 27.Weeda ER, AlDoughaim M, Criddle S. Association Between Potentially Inappropriate Medications and Hospital Encounters Among Older Adults: A Meta-Analysis. Drugs Aging. 2020. Jul;37(7):529–37. [DOI] [PubMed] [Google Scholar]
- 28.Wang P, Wang Q, Li F, Bian M, Yang K. Relationship Between Potentially Inappropriate Medications And The Risk Of Hospital Readmission And Death In Hospitalized Older Patients. Clin Interv Aging. 2019;14:1871–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paque K, Elseviers M, Vander Stichele R, Dilles T, Pardon K, Deliens L, et al. Associations of potentially inappropriate medication use with four year survival of an inception cohort of nursing home residents. Arch Gerontol Geriatr. 2019. Feb;80:82–7. [DOI] [PubMed] [Google Scholar]
- 30.do Nascimento MMG, de M Mambrini JV, Lima-Costa MF, Firmo JOA, Peixoto SWV, de Loyola Filho AI. Potentially inappropriate medications: predictor for mortality in a cohort of community-dwelling older adults. Eur J Clin Pharmacol. 2017. May;73(5):615–21. [DOI] [PubMed] [Google Scholar]
- 31.Muhlack DC, Hoppe LK, Weberpals J, Brenner H, Schöttker B. The Association of Potentially Inappropriate Medication at Older Age With Cardiovascular Events and Overall Mortality: A Systematic Review and Meta-Analysis of Cohort Studies. J Am Med Dir Assoc. 2017. Mar;18(3):211–20. [DOI] [PubMed] [Google Scholar]
- 32.Hyttinen V, Jyrkkä J, Saastamoinen LK, Vartiainen AK, Valtonen H. The association of potentially inappropriate medication use on health outcomes and hospital costs in community-dwelling older persons: a longitudinal 12-year study. Eur J Health Econ. 2019. Mar 1;20(2):233–43. [DOI] [PubMed] [Google Scholar]
- 33.Chang TI, Park H, Kim DW, Jeon EK, Rhee CM, Kalantar-Zadeh K, et al. Polypharmacy, hospitalization, and mortality risk: a nationwide cohort study. Sci Rep. 2020. Nov 3;10(1):18964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beers MH, Ouslander JG, Rollingher I, Reuben DB, Brooks J, Beck JC. Explicit criteria for determining inappropriate medication use in nursing home residents. UCLA Division of Geriatric Medicine. Arch Intern Med. 1991. Sep;151(9):1825–32. [PubMed] [Google Scholar]
- 35.Dyer SM, Crotty M, Fairhall N, Magaziner J, Beaupre LA, Cameron ID, et al. A critical review of the long-term disability outcomes following hip fracture. BMC Geriatr. 2016. Sep 2;16(1):158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gill TM, Murphy TE, Gahbauer EA, Allore HG. Association of Injurious Falls With Disability Outcomes and Nursing Home Admissions in Community-Living Older Persons. Am J Epidemiol. 2013. Aug 1;178(3):418–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xie Y, Bowe B, Yan Y, Xian H, Li T, Al-Aly Z. Estimates of all cause mortality and cause specific mortality associated with proton pump inhibitors among US veterans: cohort study. BMJ [Internet]. 2019. May 30 [cited 2019 Dec 18];365. Available from: https://www.bmj.com/content/365/bmj.l1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maggio M, Corsonello A, Ceda GP, Cattabiani C, Lauretani F, Buttò V, et al. Proton Pump Inhibitors and Risk of 1-Year Mortality and Rehospitalization in Older Patients Discharged From Acute Care Hospitals. JAMA Intern Med. 2013. Apr 8;173(7):518–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu J, Dickinson S, Elgebaly Z, Blogg S, Heaney A, Soo Y, et al. Impact of NPS MedicineWise general practitioner education programs and Choosing Wisely Australia recommendations on prescribing of proton pump inhibitors in Australia. BMC Fam Pract. 2020. May 9;21(1):85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daniels B, Pearson SA, Buckley NA, Bruno C, Zoega H. Long-term use of proton-pump inhibitors: whole-of-population patterns in Australia 2013–2016. Ther Adv Gastroenterol. 2020. Mar 19;13:1756284820913743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Devraj R, Deshpande M. Demographic and health-related predictors of proton pump inhibitor (PPI) use and association with chronic kidney disease (CKD) stage in NHANES population. Res Soc Adm Pharm. 2020. Jun 1;16(6):776–82. [DOI] [PubMed] [Google Scholar]
- 42.Raoul JL, Guérin-Charbonnel C, Edeline J, Simmet V, Gilabert M, Frenel JS. Prevalence of Proton Pump Inhibitor Use Among Patients With Cancer. JAMA Netw Open. 2021. Jun 16;4(6):e2113739–e2113739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hálfdánarson ÓÖ, Pottegård A, Björnsson ES, Lund SH, Ogmundsdottir MH, Steingrímsson E, et al. Proton-pump inhibitors among adults: a nationwide drug-utilization study. Ther Adv Gastroenterol. 2018. May 30;11:1756284818777943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lockery JE, Collyer TA, Reid CM, Ernst ME, Gilbertson D, Hay N, et al. Overcoming challenges to data quality in the ASPREE clinical trial. Trials. 2019. Dec 9;20(1):686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steinman MA, Rosenthal GE, Landefeld CS, Bertenthal D, Kaboli PJ. Agreement between drugs-to-avoid criteria and expert assessments of problematic prescribing. Arch Intern Med. 2009. Jul 27;169(14):1326–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Medications included and excluded as PIMs for this analysis based on modified AGS Beers Criteria.
Table S2: Baseline PIMs prevalence by medication class.
Table S3: Risk of disability and hospitalization outcomes by baseline PIMs exposure, in participants who experienced both events.
Table S4: Assessment of interaction between Cox models for PIMs and Polypharmacy.
Table S5: Risk of primary endpoint, death, dementia, disability and hospitalisation outcomes by baseline PIMs medication category exposure.
Table S6: Risk of cause of death from specific causes by baseline PPI use.