Abstract
Background:
To determine if altered serum lipid metabolites are associated with the risk of relapse and disability in children with multiple sclerosis (MS).
Methods:
We collected serum samples from 61 participants with pediatric-onset MS within four years of disease onset. Prospective longitudinal relapse data and cross-sectional disability measures (Expanded Disability Status Scale [EDSS]) were collected. Serum metabolomics was performed using untargeted liquid chromatography and mass spectrometry. Individual lipid metabolites were clustered into pre-defined pathways. The associations between clusters of metabolites and relapse rate and EDSS score were estimated utilizing negative binomial and linear regression models, respectively.
Results:
We found that serum acylcarnitines (relapse rate: normalized enrichment score [NES]=2.1, q=1.03E-04; EDSS: NES=1.7, q=0.02) and poly-unsaturated fatty acids (relapse rate: NES=1.6, q=0.047; EDSS: NES=1.9, q=0.005) were associated with higher relapse rates and EDSS, while serum phosphatidylethanolamines (relapse rate: NES=−2.3, q=0.002; EDSS: NES=−2.1, q=0.004), plasmalogens (relapse rate: NES=−2.5, q=5.81E-04; EDSS: NES=−2.1, q=0.004), and primary bile acid metabolites (relapse rate: NES=−2.0, q=0.02; EDSS: NES=−1.9, q=0.02) were associated with lower relapse rates and lower EDSS.
Conclusions:
This study supports the role of some lipid metabolites in pediatric MS relapses and disability.
Keywords: pediatric onset MS, lipidomics, relapse rate, EDSS
INTRODUCTION
Multiple sclerosis (MS) is a chronic demyelinating disease of the central nervous system (CNS) with congruent inflammatory and degenerative elements and of autoimmune origin.1 Metabolomics – which involves defining the concentration of small molecules in various biological matrices – could provide a better understanding of the pathophysiology of the disease since they can reflect and summarize information from other “omics” techniques in addition to their unique insight.2 The study of the metabolome in MS has been rapidly growing during recent years due to advancing technology for measuring various metabolites and a need for biomarkers for diagnosis, prognosis, and treatment response. We have previously shown that the circulating metabolome is different in MS compared to healthy controls in both adult and pediatric populations.3,4 Whether these differences are part of the pathogenesis of MS, a byproduct of the disease process and inflammation, or driven by different environmental factors is not known.
Lipid metabolites are of particular interest not only because they are the major structural component of myelin but also due to their prominent role in brain function, neuroinflammation, and cell signaling.5,6 The importance of lipids has also been highlighted in a dietary study that demonstrated an association of different lipid intake with MS activity, with a diet high in saturated fat being associated with a higher relapse rate.7 Understanding the role of lipids in MS can potentially result in identifying new therapeutic strategies.
While relatively rare, pediatric-onset MS (POMS) can serve as a unique platform to investigate lipid’s role in the disease course. The limited exposure to environmental and other confounding factors, such as comorbidities, along with the higher relapse rates8 in this group may provide better insight into disease mechanisms. This study aimed to determine if serum levels of various lipids contribute to the disease activity and severity.
METHODS
Patient recruitment
The Pediatric MS Center at the University of California, San Francisco (UCSF) served as the recruitment site for MS cases from January 2006 to November 2011. Patients with POMS or clinically isolated syndrome (CIS) at risk of MS with at least two silent T2-bright foci on magnetic resonance imaging (MRI) with clinical onset before age 18 years and seen within four years of symptom onset were enrolled. Patients with myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD) were excluded from analyses. UCSF IRB approved the study protocol, and parental consent and child assent were obtained before enrollment in the study.
Demographic and clinical information
Demographic data were obtained at the time of study enrollment. Race and ethnicity were categorized into the following groups: (1) White, non-Hispanic, (2) White, Hispanic, (3) Black, and (4) others. National Institute of Health criteria was used for reporting self-identified race and ethnicity (https://grants.nih.gov/grants/guide/notice-files/not-od-15-089.html).
Data were entered prospectively in a database after each clinic visit, including time of relapses, use and start/stop time of disease-modifying therapies (DMTs), and Expanded Disability Status Scale (EDSS) score. Blood collection occurred in the morning after the clinic visit, during which EDSS was recorded. The fasting state of the subjects was not recorded. Serum was isolated following a standardized protocol and stored at −80°C within three hours of collection until metabolomics analyses. Relapses were defined as new or recurrent neurological symptoms localizing to the CNS that lasted for a minimum of 24 h after remission of at least 30 days since the previous attack in the absence of fever or infection. For the analyses, only relapses occurring after study enrollment were included. The duration of follow-up was calculated from the time of enrollment to the last available follow-up visit. The use of disease-modifying therapy (DMT) was reported as ‘exposed versus unexposed,’ with ‘exposed’ defined as >50% of the time on a DMT during study follow-up.
Untargeted metabolomics analysis
Serum global untargeted metabolomics utilizing liquid chromatography followed by tandem mass spectrometry was performed as previously described (Metabolon Inc., Morrisville, NC).9 Briefly, proteins were precipitated, and samples were divided into five fractions for analyses by different mass spectrometry techniques. Metabolites were identified by automated comparison of ion features in the study samples to a reference library. After calculating peak areas, raw values for each metabolite were normalized to the sum of all measured peak areas in that sample (mTIC normalization). Lipid metabolites were identified through this method, and then regrouped into categories based on their metabolic pathways for analysis.
Statistical analyses
Metabolites were measured in two metabolomics runs consisting of 26 and 35 samples. There were 778 common metabolites between the two runs. Metabolites with ≥30% of missing values were excluded (n=98). Missing values were imputed utilizing k-nearest neighbors using the R package impute (neighboring samples were identified by calculating Euclidean distance between metabolites; 3 neighbors were used for each imputation). Consistent results were observed in a sensitivity analysis using the minimum value of the observed metabolites across samples to impute the missing values. Metabolite concentrations were then normalized by log-transformation. ComBat algorithm was used to adjust for batch effect, using the R package sva, and the two runs were combined.10 No outliers were detected using a Euclidean distance-based sample network as suggested by Langfelder and Horvath.11 Since our study was focused on lipids, we limited the analyses to 370 lipid metabolites. Metabolites were then scaled by dividing by their standard deviation.
For every metabolite, we evaluated the impact of its levels on the two outcome variables (relapse incidence rate ratio and baseline EDSS). To achieve that, we modeled the number of relapses using negative binomial regression and the EDSS score using linear terms. Both models were adjusted for sex, age, race, DMT status (exposed versus unexposed), and vitamin D serum levels. In the relapse model, log-transformed years of follow-up were used as an offset term. Beta coefficients derived from the relapses model were exponentiated to get the relapse incidence rate ratio (IRR).
Because of the relatively small sample size and to make the results more robust, we performed a metabolic pathway-based analysis. To achieve this, we classified metabolites into biologically meaningful pathways (consisting of >3 metabolites) based on the classification that Metabolon provided (e.g., endocannabinoids, ceramides, plasmalogens) (Supplementary Table). We then performed a metabolite set enrichment analysis (MSEA)12, which follows the same steps as the gene set enrichment analysis13 using the R package fgsea. In brief, all metabolites are ordered by a correlation with a phenotype, and a normalized enrichment score (NES) is calculated for each pre-defined pathway based on the size of the pathway and the degree to which that pathway’s metabolites are overrepresented at the extremes (positives or negatives) of the entire ranked list of metabolites. We performed MSEA separately for our two outcomes (relapses and EDSS). We considered the beta coefficient estimated from the respective models (described above) divided by its standard error as metric supplied to MSEA in order to rank the metabolites. Pathways comprising three or fewer metabolites were excluded from the analysis, resulting in 25 final pathways.
All statistical analyses were performed using R Version 4.2.0 (https://www.r-project.org/). Statistical significance was defined as p<0.05 and false discovery rate-adjusted q<0.05.
RESULTS
Baseline demographics and clinical characteristics
Demographic and clinical characteristics at the time of enrollment for the 61 MS/CIS patients are summarized in Table 1. The average follow-up time was 2.8 years after enrollment in the study.
Table 1.
Demographics and clinical characteristics
| Baseline Characteristics | N = 61 |
|---|---|
| Female, n (%) | 28 (45%) |
| Age, Mean (SD) | 14.6 (2.7) |
| Race/ethnicity, n (%) | |
| White, non-Hispanic | 31 (51%) |
| White, Hispanic | 20 (33%) |
| African American | 5 (8%) |
| Other | 5 (8%) |
| Disease duration, years, median (IQR) | 0.5 (0.2 – 1.2) |
| DMT exposed, n (%) | 45 (74%) |
| 25(OH) Vitamin D in ng/mL, Mean (SD) | 23.3 (8.8) |
| EDSS, Median (IQR) | 1.5 (1.0 – 2.0) |
| Follow-up duration in years, Median (IQR) | 2.8 (1.7 – 4.9) |
| Number of relapses during follow-up, Median (IQR) | 1 (0 – 2) |
Association between serum lipid metabolites and risk of MS relapse and EDSS
Results from the individual metabolite analyses are provided in the Supplementary Table.
Table 2 shows each lipid pathway’s NES of relapse IRR and EDSS. Positive enrichment scores indicate a positive association with the outcome, while negative NES suggests a negative association. Acylcarnitines (relapse IRR: NES=2.1, q=1.0E-04; EDSS: NES=1.7, q=0.02) and poly-unsaturated fatty acids (PUFAs) (relapse IRR: NES=1.6, q=0.047; EDSS: NES=1.9, q=0.005)were significantly associated with an increased risk of relapse and worse baseline EDSS scores. On the other hand, phosphatidylethanolamines (PEs) (relapse IRR: NES=−2.3, q=0.002; EDSS: NES=−2.1, q=0.004), plasmalogens (relapse IRR: NES=−2.5, q=5.81E-04; EDSS: NES=−2.1, q=0.004), and primary bile acids (relapse IRR: NES=−2.0, q=0.02; EDSS: NES=−1.9, q=0.02) were associated with a lower risk of relapse and lower baseline EDSS scores. Additionally, ceramides (NES=2.1, q=8.96E-04), sphingolipid metabolites (NES=1.6, q=0.03), monoacylglycerols (NES=1.8, q=0.02), and diacylglycerols (DAGs) (NES=1.8, q=0.02) were significantly associated with worse baseline EDSS scores. Monohydroxy fatty acids were also associated with an increased risk of relapses (NES=1.9, q=0.003), while phosphatidylcholines (PCs) (NES=−1.8, q=0.03). Dicarboxylate fatty acids were associated with a higher risk of relapses (NES=1.6, q=0.048), but lower baseline EDSS scores (NES=−2.5, q=7.47E-05). Figure 1 demonstrates the individual metabolites of selected significant pathways related to relapse rate and EDSS. Figures of all the pathways can be found in the Supplementary File.
Table 2.
Without the DDNOS participant
| Pathway | Pathway size | Relapse IRR | EDSS | ||||
|---|---|---|---|---|---|---|---|
| NES | p-value | q-value | NES | p-value | q-value | ||
| Androgenic Steroids | 15 | 1.4 | 0.06 | 0.13 | −0.8 | 0.77 | 0.85 |
| Ceramides | 19 | 1.4 | 0.07 | 0.15 | 2.1 | 7.17E-05 | 8.96E-04 |
| Diacylglycerol | 26 | 0.8 | 0.71 | 0.81 | 1.8 | 0.004 | 0.02 |
| Endocannabinoid | 4 | 1.3 | 0.11 | 0.19 | 0.9 | 0.69 | 0.80 |
| Fatty Acid Metabolism (Acyl Choline) | 7 | −2.2 | 2.84E-04 | 0.002 | −0.9 | 0.64 | 0.78 |
| Fatty Acid Metabolism (Acyl Carnitine) | 29 | 2.1 | 4.11E-06 | 1.03E-04 | 1.7 | 0.006 | 0.02 |
| Fatty Acid, Dicarboxylate | 19 | 1.6 | 0.02 | 0.048 | −2.5 | 1.49E-06 | 7.47E-05 |
| Fatty Acid, Monohydroxy | 16 | 1.9 | 3.68E-04 | 0.003 | −0.9 | 0.57 | 0.71 |
| Long Chain Fatty Acid | 14 | 1.6 | 0.02 | 0.04 | 1.4 | 0.08 | 0.15 |
| Lysophospholipid | 29 | −1.0 | 0.37 | 0.50 | −0.6 | 0.96 | 0.96 |
| Lysoplasmalogen | 4 | −1.5 | 0.10 | 0.18 | −1.3 | 0.15 | 0.23 |
| Medium Chain Fatty Acid | 7 | 1.4 | 0.08 | 0.15 | −0.6 | 0.92 | 0.94 |
| Monoacylglycerol | 9 | −1.6 | 0.05 | 0.12 | 1.8 | 0.004 | 0.02 |
| Phosphatidylcholine (PC) | 18 | −1.8 | 0.01 | 0.03 | −0.8 | 0.78 | 0.85 |
| Phosphatidylethanolamine (PE) | 11 | −2.3 | 2.12E-04 | 0.002 | −2.1 | 6.68E-04 | 0.004 |
| Phosphatidylinositol (PI) | 6 | −1.5 | 0.07 | 0.15 | −1.2 | 0.25 | 0.37 |
| Phospholipid Metabolism | 7 | −0.8 | 0.67 | 0.80 | 0.7 | 0.80 | 0.85 |
| Plasmalogen | 11 | −2.5 | 3.49E-05 | 5.81E-04 | −2.1 | 6.92E-04 | 0.004 |
| Polyunsaturated Fatty Acid (n3 and n6) | 14 | 1.6 | 0.02 | 0.047 | 1.9 | 9.08E-04 | 0.005 |
| Pregnenolone Steroids | 5 | 1.1 | 0.36 | 0.50 | −1.4 | 0.11 | 0.19 |
| Primary Bile Acid Metabolism | 9 | −2.0 | 0.006 | 0.02 | −1.9 | 0.005 | 0.02 |
| Progestin Steroids | 5 | 0.7 | 0.88 | 0.92 | −1.7 | 0.03 | 0.07 |
| Secondary Bile Acid Metabolism | 11 | −0.9 | 0.54 | 0.71 | −1.3 | 0.13 | 0.20 |
| Sphingolipid Metabolism | 42 | 1.0 | 0.56 | 0.71 | 1.6 | 0.01 | 0.03 |
| Sterol | 7 | −1.4 | 0.13 | 0.20 | −1.1 | 0.37 | 0.50 |
Normalized enrichment scores from metabolite set enrichment analysis derived for association with relapse rate and EDSS scores. Positive values denote positive association of metabolite levels and outcomes, while negative values denote negative association.
Metabolites that are of statistical significance are bolded.
Figure 1.
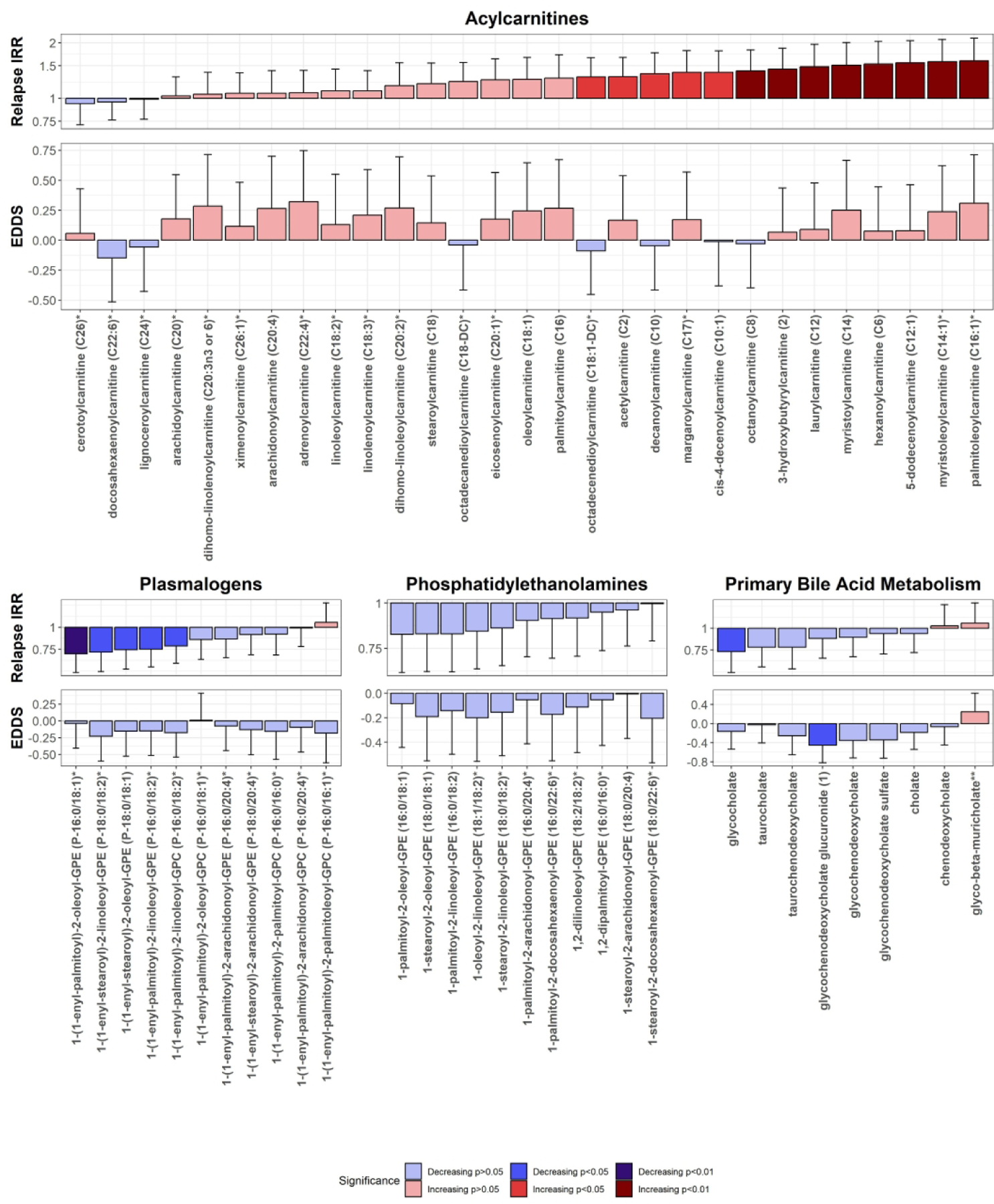
Individual results for metabolites in selected pathways in MSEA demonstrated a significant correlation with relapse rate and EDSS scores. X-axis denotes the metabolites included in the particular pathway; Y axis denotes the change in the relapse rate ratio or the EDSS score for a 1 SD increase in the metabolite levels. Whiskers denote the one-sided 95% interval of the metabolite direction. Bars are colored based on the direction and level of significance in individual metabolite analyses.
IRR=incidence rate ratio
DISCUSSION
Alterations in lipid metabolism and their effects on various immune cells have been reported in patients with relapsing and progressive forms of MS.14 Differences in the cerebrospinal fluid lipidomics between people with MS, and healthy controls have also been described.15 Our investigation of serum lipid metabolites in pediatric MS suggests several lipid pathways may be associated with MS activity and disability. These lipid metabolites may have a direct detrimental role in the inflammatory and neurodegenerative mechanisms. They can also be a consequence and downstream effect of the pathological changes occurring in the disease (myelin degradation and axonal injury) or be part of a compensatory, protective effort in the body. These associations may evolve and change at various stages of the disease (as has been reported in animal models of neuroinflammation).16
Most lipidomics studies in MS have focused on the differences between people with MS and healthy controls. The association between serum and plasma lipidomics and disease activity or disability status has not been studied extensively. In this study, we focused on the two important, and relevant clinical outcomes in MS that are usually the primary outcomes of phase 3 clinical trials in relapsing-remitting MS. Relapse rate reflects MS clinical activity and indicates the severity of adaptive immune-mediated demyelination. EDSS, on the other hand, reflects not only the severity of immune-mediated demyelination and neurodegeneration in the CNS but also the success of repair and compensatory mechanisms. So, factors can be associated with both relapse rate and disability as measured by the EDSS, and some may affect one or the other. In our study, the acylcarnitine and PUFA pathway was associated with higher relapse rates and higher EDSS, while PE, plasmalogen, and primary bile acid metabolism pathways were associated with lower relapse rates and lower EDSS. The acylcholine, long-chain fatty acid, and monohydroxy fatty acid pathways were only associated with a higher relapse rate. In contrast, the ceramide, sphingolipid metabolism, monoacylglycerol, and DAG pathways were associated with higher EDSS, and the dicarboxylate fatty acid pathway was associated with lower EDSS.
Several ceramides, especially the long- and very-long-chain ones, have been reported to be associated with oligodendrocyte injury and cell death.6,17 Increased levels of C24 ceramide were associated with impaired mitochondrial function in neurons in vitro, resulting in decreased energy production and, eventually, axonal damage.18 In two case-control studies, the levels of ceramides in blood and white blood cells differed between people with MS and healthy controls.19,20 Increased levels of serum long- and very-long-chain ceramides were associated with more rapid disability progression and faster retinal atrophy.20 In another study, increased levels of ceramides were found in the CSF of people with MS compared to healthy controls, and ceramides were sufficient to induce oxidative damage to neurons.21 Ceramides are also involved in the apoptosis process induced by inflammatory cytokines.22 The amount of ceramides is highly connected to sphingomyelins and sphingosines levels through the sphingomyelin synthase/sphingomyelinase and ceramidase/ceramide synthase paths, respectively.6 This may explain why the effects of these two pathways on the clinical outcomes are in the same direction. Our observation of the association of the serum ceramide pathway – which only included long- and very-long-chain ceramides – with higher relapse rate and EDSS is in line with the previous report on the importance of these metabolites in neuroinflammatory demyelination.
Acylcarnitines are important metabolites in cellular energy metabolism pathways, particularly beta-oxidation.23 Reduction in some acylcarnitines has been reported as part of the metabolic changes associated with MS.24–26 In contrast with these results, studies of other systemic diseases, like diabetes, have found acylcarnitines to be elevated in these states as a possible result of mitochondrial dysfunction and impaired fatty acid oxidation.27 It has also been shown that medium-chain acylcarnitines (C8-C14) can induce the production of proinflammatory cytokines.28 Our observation of the association of higher relapse rate and EDSS with the serum acylcarnitine pathway – which is mainly driven by the medium-chain ones - may either denote a compensatory response or the aftermath of the inflammatory response and mitochondrial dysfunction associated with a demyelinating attack. We cannot rule out that it could also be related to a change in patients’ dietary habits after receiving the MS diagnosis, which is unlikely in pediatric MS.
PEs and PCs are phospholipids that, besides being a structural component of the cell membranes, can also modulate the immune response.29 Some members of PEs have been shown to be different between people with MS and healthy control.30 Ether PEs have been reported to be lower in the plasma of twins discordant for MS.31 In another study, PEs and other phospholipids like phosphatidylcholines (PCs), were found to be decreased in MS, while DAGs, which are degradation products of these lipids, were found to be increased, suggesting a high turnover of these phospholipids.29 Additionally, reduced PEs are consistent with disruptions in energy metabolism.32 In this cohort, we found that increasing levels of PEs and PCs were associated with lower relapse rates. Phosphatidylinositols, another class of phospholipids, were also trending in the same direction. Higher PE levels were also associated with lower EDSS scores while increasing levels of DAGs were associated with worse EDSS scores. These findings support the hypothesis of increased phospholipid turnover and suggest that it is associated with increased disease activity.
Plasmalogens are another membrane component and are highly expressed in the nervous system.33 Besides their structural role, they have antioxidant and neuroprotective properties, mainly due to their free radical scavenging abilities.34 This is more prominent in the PE plasmalogens in comparison to PC plasmalogens. Plasmalogens have been found to be decreased in the setting of neuroinflammation due to increased oxidative stress, as well as increased ceramide levels which activate the plasmalogen-specific phospholipase A2.34 Our results are congruent with these PE properties since we found them to be associated with decreased risk of relapses and decreased EDSS scores.
PUFAs have been extensively studied over recent years regarding neuroinflammation. Although omega-3 (n3) PUFAs have been generally associated with neuroprotection and omega-6 (n6) with worsening neuroinflammation through the arachidonic acid pathways, the reality might be more complicated.35,36 In our study, the PUFA pathway consisted of both n3 and n6 PUFA metabolites, with the majority of them being of the n6 class. Thus, the direction of the relationship between this pathway and the clinical outcomes was mainly driven by the n6 component, higher levels of which were associated with more relapses and worse EDSS.
Interestingly, the dicarboxylate fatty acid pathway was associated with more relapses but lower EDSS scores. The components of this pathway have not been studied in association with neuroinflammation, demyelination, or neurodegeneration. The expectation that a factor associated with a higher relapse rate be associated with a more severe disability is not necessarily correct. The relapse rate reflects the intensity of adaptive immune-mediated inflammation. The severity of disability (or the EDSS score) not only reflects the extent of immune-mediated demyelination and neurodegeneration but also is affected by repair, remyelination, neural plasticity, and reserve. So, it is conceivable that a factor leading to more severe inflammation is associated with better repair and remyelination capacity.
Our group has previously reported that the blood levels of circulating bile acids are lower in adult and pediatric patients with MS compared to controls and showed that supplementation with bile acids could reduce neuronal injury in a mouse model of neuroinflammation.37 Reduced bile acid production is consistent with an oxidative stress-induced cholestasis.38 Here, we report that the primary bile acid pathway is associated with a lower relapse rate and disability in patients with pediatric MS. This observation strengthens the possibility that some bile acids may have a protective role in MS and provides further support for performing intervention trials in this disease.
Our study has several strengths. Pediatric patients are exposed to fewer environmental factors and potentially have fewer non-relevant risk factors and comorbidities affecting the disease course and measured metabolites. Patients participating in this study underwent stringent case ascertainment, and people with other neuroinflammatory diseases (such as MOGAD) were excluded. Enrollment occurred shortly after disease onset (median of 0.5 years after clinical onset). Relapses were prospectively captured (after study enrollment). Serum was prepared rapidly after blood collection and frozen at −80°C. We adequately addressed the batch effect and adjusted our statistical models for potential confounders, such as serum 25-OH vitamin D levels and the use of DMTs.
The study also has some important limitations. Blood samples were collected after the MS diagnosis, and it is possible that patients changed their diets based on their perceived disease severity. That said, there is no universally accepted dietary change advice for MS patients. The fasting status of the participants at the time of blood draw for the metabolomic analyses was not recorded, and it could have affected some blood lipid metabolite levels. Body mass index data, which could potentially influence some lipid metabolites’ levels, were not collected. MRI data were unavailable to inform on subclinical disease activity, and the EDSS scores were not confirmed at a follow-up visit. In addition, we could not identify another independent data/sample set to replicate our results. Finally, the current project is an observational study, and the reported associations should not be interpreted as causal, and unknown and unmeasured confounders may have affected the results. Furthermore, the metabolomic signature could change during development, and these results may not apply to adult MS.
Overall, our results support the role of several lipid metabolic pathways in the course of MS. Lipid metabolites should be further investigated as biomarkers of disease activity and severity, as findings could lead to identifying new therapeutic targets.
Supplementary Material
FUNDING
This work was supported by an NIH grant (EW, R01NS071463), a grant from Race to Erase MS (EW), and a grant from the National MS Society (NMSS) (EW, RG-1701-26635).
Additional support was provided to JWN by USDA Project 2032-51530-025-00D. The USDA is an equal opportunity provider and employer.
Footnotes
DECLARATION OF CONFLICTING INTERESTS
The Authors declare that there is no conflict of interest.
REFERENCES
- 1.Reich DS, Lucchinetti CF, Calabresi PA. Multiple Sclerosis. N Engl J Med 2018; 378: 169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhargava P, Anthony DC. Metabolomics in multiple sclerosis disease course and progression. Mult Scler J 2020; 26: 591–598. [DOI] [PubMed] [Google Scholar]
- 3.Fitzgerald KC, Smith MD, Kim S, et al. Multi-omic evaluation of metabolic alterations in multiple sclerosis identifies shifts in aromatic amino acid metabolism. Cell Rep Med 2021; 2: 100424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nourbakhsh B, Bhargava P, Tremlett H, et al. Altered tryptophan metabolism is associated with pediatric multiple sclerosis risk and course. Ann Clin Transl Neurol 2018; 5: 1211–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stadelmann C, Timmler S, Barrantes-Freer A, et al. Myelin in the Central Nervous System: Structure, Function, and Pathology. Physiol Rev 2019; 99: 1381–1431. [DOI] [PubMed] [Google Scholar]
- 6.Hannun YA, Obeid LM. Sphingolipids and their metabolism in physiology and disease. Nat Rev Mol Cell Biol 2018; 19: 175–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Azary S, Schreiner T, Graves J, et al. Contribution of dietary intake to relapse rate in early paediatric multiple sclerosis. J Neurol Neurosurg Psychiatry 2018; 89: 28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gorman MP, Healy BC, Polgar-Turcsanyi M, et al. Increased Relapse Rate in Pediatric-Onset Compared With Adult-Onset Multiple Sclerosis. Arch Neurol 2009; 66: 54–59. [DOI] [PubMed] [Google Scholar]
- 9.Evans AM, DeHaven CD, Barrett T, et al. Integrated, Nontargeted Ultrahigh Performance Liquid Chromatography/Electrospray Ionization Tandem Mass Spectrometry Platform for the Identification and Relative Quantification of the Small-Molecule Complement of Biological Systems. Anal Chem 2009; 81: 6656–6667. [DOI] [PubMed] [Google Scholar]
- 10.Leek JT, Johnson WE, Parker HS, et al. The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics 2012; 28: 882–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics 2008; 9: 559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fitzgerald KC, Bhargava P, Smith MD, et al. Intermittent calorie restriction alters T cell subsets and metabolic markers in people with multiple sclerosis. eBioMedicine; 82. Epub ahead of print 1 August 2022. DOI: 10.1016/j.ebiom.2022.104124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci 2005; 102: 15545–15550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pineda-Torra I, Siddique S, Waddington KE, et al. Disrupted Lipid Metabolism in Multiple Sclerosis: A Role for Liver X Receptors? Front Endocrinol; 12, https://www.frontiersin.org/articles/10.3389/fendo.2021.639757 (2021, accessed 1 September 2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nogueras L, Gonzalo H, Jové M, et al. Lipid profile of cerebrospinal fluid in multiple sclerosis patients: a potential tool for diagnosis. Sci Rep 2019; 9: 11313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee G, Hasan M, Kwon O-S, et al. Identification of Altered Metabolic Pathways during Disease Progression in EAE Mice via Metabolomics and Lipidomics. Neuroscience 2019; 416: 74–87. [DOI] [PubMed] [Google Scholar]
- 17.Kim S, Steelman AJ, Zhang Y, et al. Aberrant Upregulation of Astroglial Ceramide Potentiates Oligodendrocyte Injury. Brain Pathol 2012; 22: 41–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wentling M, Lopez-Gomez C, Park H-J, et al. A metabolic perspective on CSF-mediated neurodegeneration in multiple sclerosis. Brain 2019; 142: 2756–2774. [DOI] [PubMed] [Google Scholar]
- 19.Kurz J, Brunkhorst R, Foerch C, et al. The relevance of ceramides and their synthesizing enzymes for multiple sclerosis. Clin Sci 2018; 132: 1963–1976. [DOI] [PubMed] [Google Scholar]
- 20.Filippatou AG, Moniruzzaman M, Sotirchos ES, et al. Serum ceramide levels are altered in multiple sclerosis. Mult Scler J 2021; 27: 1506–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vidaurre OG, Haines JD, Katz Sand I, et al. Cerebrospinal fluid ceramides from patients with multiple sclerosis impair neuronal bioenergetics. Brain 2014; 137: 2271–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scurlock B, Dawson G. Differential responses of oligodendrocytes to tumor necrosis factor and other pro-apoptotic agents: Role of ceramide in apoptosis. J Neurosci Res 1999; 55: 514–522. [DOI] [PubMed] [Google Scholar]
- 23.Dambrova M, Makrecka-Kuka M, Kuka J, et al. Acylcarnitines: Nomenclature, Biomarkers, Therapeutic Potential, Drug Targets, and Clinical Trials. Pharmacol Rev 2022; 74: 506–551. [DOI] [PubMed] [Google Scholar]
- 24.Andersen SL, Briggs FBS, Winnike JH, et al. Metabolome-based signature of disease pathology in MS. Mult Scler Relat Disord 2019; 31: 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kasakin MF, Rogachev AD, Predtechenskaya EV, et al. Targeted metabolomics approach for identification of relapsing-remitting multiple sclerosis markers and evaluation of diagnostic models. MedChemComm 2019; 10: 1803–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng D, An Z, Ge M, et al. Medium & long-chain acylcarnitine’s relation to lipid metabolism as potential predictors for diabetic cardiomyopathy: a metabolomic study. Lipids Health Dis 2021; 20: 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCann MR, George De la Rosa MV, Rosania GR, et al. L-Carnitine and Acylcarnitines: Mitochondrial Biomarkers for Precision Medicine. Metabolites 2021; 11: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rutkowsky JM, Knotts TA, Ono-Moore KD, et al. Acylcarnitines activate proinflammatory signaling pathways. Am J Physiol - Endocrinol Metab 2014; 306: E1378–E1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Villoslada P, Alonso C, Agirrezabal I, et al. Metabolomic signatures associated with disease severity in multiple sclerosis. Neurol - Neuroimmunol Neuroinflammation 2017; 4: e321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferreira HB, Melo T, Monteiro A, et al. Serum phospholipidomics reveals altered lipid profile and promising biomarkers in multiple sclerosis. Arch Biochem Biophys 2021; 697: 108672. [DOI] [PubMed] [Google Scholar]
- 31.Penkert H, Lauber C, Gerl MJ, et al. Plasma lipidomics of monozygotic twins discordant for multiple sclerosis. Ann Clin Transl Neurol 2020; 7: 2461–2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calzada E, Onguka O, Claypool SM. Phosphatidylethanolamine Metabolism in Health and Disease. Int Rev Cell Mol Biol 2016; 321: 29–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wallner S, Schmitz G. Plasmalogens the neglected regulatory and scavenging lipid species. Chem Phys Lipids 2011; 164: 573–589. [DOI] [PubMed] [Google Scholar]
- 34.Bozelli JC, Azher S, Epand RM. Plasmalogens and Chronic Inflammatory Diseases. Front Physiol; 12, https://www.frontiersin.org/articles/10.3389/fphys.2021.730829 (2021, accessed 13 March 2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bazinet RP, Layé S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat Rev Neurosci 2014; 15: 771–785. [DOI] [PubMed] [Google Scholar]
- 36.Farooqui AA, Horrocks LA, Farooqui T. Modulation of inflammation in brain: a matter of fat. J Neurochem 2007; 101: 577–599. [DOI] [PubMed] [Google Scholar]
- 37.Bhargava P, Smith MD, Mische L, et al. Bile acid metabolism is altered in multiple sclerosis and supplementation ameliorates neuroinflammation. J Clin Invest 2020; 130: 3467–3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roma MG, Sanchez Pozzi EJ. Oxidative stress: A radical way to stop making bile. Ann Hepatol 2008; 7: 16–33. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


