Abstract
Background
Cardiopulmonary failure is the leading cause of death in Duchenne muscular dystrophy (DMD). Research into DMD-specific cardiovascular therapies is ongoing, but there are no FDA-approved cardiac endpoints. To adequately power a therapeutic trial, appropriate endpoints must be chosen and the rate of change for these endpoints reported. The objective of this study was to evaluate rate of change for cardiac magnetic resonance (CMR) and blood biomarkers and to determine which measures associate with all-cause mortality in DMD.
Methods
Seventy-eight DMD subjects underwent 211 CMRs analyzed for left ventricular ejection fraction (LVEF), indexed LV end diastolic and systolic volumes, circumferential strain (Ecc), late gadolinium enhancement (LGE) presence and severity (global severity score, GSS, and full-width half maximum, FWHM), native T1 mapping, T2 mapping, and extracellular volume (). Blood samples were analyzed for brain natriuretic peptide (BNP), n-terminal proBNP (NTproBNP), and troponin I. Cox proportional hazard regression modeling was performed with all-cause mortality as the outcome.
Results
Fifteen subjects (19%) died. LVEF, indexed end systolic volumes, GSS, and FWHM worsened at 1- and 2-years while Ecc and indexed LV end diastolic volumes worsened at 2-years. LVEF, indexed LV end diastolic and systolic volumes, LGE FWHM, and Ecc associated with all-cause mortality (p<0.05). NTproBNP was the only blood biomarker that associated with all-cause mortality (p<0.05).
Conclusions
LVEF, indexed LV volumes, Ecc, LGE FWHM, and NTproBNP are associated with all-cause mortality in DMD and may be the best endpoints for use in cardiovascular therapeutic trials. We also report change over time of CMR and blood biomarkers.
Keywords: Duchenne muscular dystrophy, Cardiomyopathy, Biomarkers, Cardiac Magnetic Resonance, All-cause Mortality
INTRODUCTION
Cardiopulmonary failure is the leading cause of mortality in Duchenne muscular dystrophy (DMD) in the current era.1 Unfortunately, standard heart failure therapies are not DMD-specific and have limited efficacy.2, 3 Drug development is ongoing but has been hampered by the lack of established and modifiable cardiovascular outcome measures for clinical trials. For maximal efficacy, most therapies should begin early in the disease process, around 10–14 years of age, if not before. Efficacy evaluation is limited by the fact that most mortality occurs in the second-third decade. Therefore, mortality alone is not an optimal outcome measure, given that trials could require up to a decade to determine improved mortality. Although the Food and Drug Administration has issued official guidance on DMD trials, they have not defined DMD-specific cardiac endpoints and have been averse to accepting surrogate outcome measures such as left ventricular ejection fraction (LVEF) and myocardial strain until stronger associations with endpoints such as mortality are established.4
A better understanding of the change in biomarkers over time is critical for power analyses when developing a clinical trial. More importantly, clinical trials must use established outcome measures that predict mortality. The objective of this study was to define progression of commonly used CMR and blood biomarkers over 1- and 2-years (the most likely length of an exploratory DMD therapeutic trial) and to evaluate whether these biomarkers associate with all-cause mortality in DMD. We hypothesized that multiple biomarkers of function and fibrosis would correlate with mortality.
METHODS
Enrollment
DMD subjects were primarily enrolled from one of two prospective observational cohorts (N=72); previously performed CMR studies were added to the database to increase datapoints. Additional DMD subjects who had signed consent for research CMR imaging but were not enrolled in those trials (N=6) were also included. The Institutional Review Board approved the studies and enrollment was completed between January 2013 and January 2020. The data that support the findings of this study are available from the corresponding author upon reasonable request. Consent was obtained for all participants; those under 18 years of age signed an age-appropriate assent form. Inclusion criteria were a diagnosis of DMD with clinical phenotype and confirmation with either genetic testing or muscle biopsy and able to tolerate CMR without sedation or anesthesia. Given challenges with breath-holds in younger children, the youngest age enrolled was 7. In order to enroll a population with a broad range of cardiovascular disease severity, no upper age limit was used for DMD patients. Exclusion criteria were additional cardiac diagnoses that could confound biomarkers and a contraindication to contrast-enhanced CMR.
Pertinent data were collected from the medical record including past medical history, medications, ambulatory status, and respiratory status (i.e. non-invasive or invasive ventilation). This included mortality, which was subsequently classified based on available information as cardiac, respiratory, infectious, or other. Cardiac mortality was defined as sudden death not preceded by other symptoms or as chronic heart failure with death at home or in hospital. Respiratory mortality was defined as acute or chronic respiratory decompensation. Infectious mortality was defined as an infectious etiology such as pneumonia or sepsis. In cases where multiple etiologies were possible, both diagnoses were coded with the primary etiology based on available information, including physician notes and the order on the death certificate when available. For example, a patient who died from pneumonia with acute respiratory decompensation and known respiratory insufficiency would be coded infectious as primary cause of death, respiratory as secondary cause of death. A patient who had known pulmonary insufficiency and progressive respiratory distress and had an elevated brain natriuretic peptide (BNP) and troponin followed by bradycardic arrest would be coded respiratory as primary cause of death and cardiac as secondary cause of death. For one patient, no information was available so subclassification was not possible. All prospectively enrolled participants provided a blood sample on day of CMR, including a hematocrit to calculate the extracellular volume . Subjects underwent multiple CMR studies as part of the protocols, the majority occurring annually. All prospectively enrolled participants also underwent quantitative muscle testing (QMT) using a handheld myometer as previously described.5 Arm QMT score (pounds) was the sum of flexion and extension values for both elbows and leg QMT was the sum of flexion and extension values for both knees; total QMT score was the sum of values for elbows and knees.
CMR Protocol
CMR was performed on a 1.5 Tesla Siemens Avanto (Siemens Healthcare Sector, Erlangen, Germany) with an 8 channel cardiac coil or a 1.5 Tesla Siemens Avanto Fit (Siemens) with a 32 channel coil. CMR protocol was performed as previously described.6 In brief, CMR protocol included functional imaging using balanced steady-state free precession imaging.7 Myocardial tagging was performed in the short axis at the base, level of the papillary muscles, and apex using a segmented k-space fast gradient echo sequence with electrocardiogram-triggering as previously described.8 T2 mapping using a breath-held, electrocardiogram (ECG)-triggered, bSSFP sequence with motion correction was performed in the short axis.
Intravenous gadolinium contrast (gadopentate dimeglumine, Magnevist®, Bayer Healthcare Pharmaceuticals, Wayne, NJ, USA at a dose of 0.2mmol/kg or gadobutrol, Gadavist®, Bayer Healthcare Pharmaceuticals, Wayne, NJ, USA at a dose of 0.15–0.2mmol/kg) was administered through a peripheral intravenous line. Late gadolinium enhancement (LGE) was performed using single shot and segmented inversion recovery (optimized inversion time to null myocardium) and phase sensitive inversion recovery (inversion time of 300ms) imaging in the 4-chamber, 3-chamber, and 2-chamber planes as well as the short axis stack.
Breath-held modified Look-Locker inversion recovery, or MOLLI, sequences were performed as previously described,6 prior to and 15 minutes after contrast administration at the base, mid-ventricular level, and apex in the short axis plane (apical slices were not analyzed due to concerns of partial volume averaging) at the same slice location as the T2-mapping and tagged images.9, 10 Modified Look-Locker inversion recovery sequences were ECG-triggered images obtained in diastole with pre-contrast acquired as a 5(3s)3 and post-contrast protocol was acquired at a 4(1)3(1)2.11 Motion correction was performed and a T1 map was generated on the scanner.12 Any image felt to be inadequate due to poor breath holds or poor motion correction was repeated at the time of the scan.
CMR Post-Processing
All CMR post-processing was performed blinded to clinical data by an image analyst with all analyses verified by an experienced cardiologist (JHS). Ventricular volumes and function were calculated using Medis QMass (MedisSuite 2.1, Medis, Leiden, The Netherlands). The presence or absence of LGE, as well as location using the standard 17-segment model,13 was qualitatively assessed. LGE severity was assessed as previously described.8 In brief, the score was calculated using all available LGE images and ranged from 0 (no LGE) to 4 (severe LGE); reproducibility of this method has been previously demonstrated.14 Percent LGE was calculated using the full width half maximum (FWHM) technique on the phase sensitive inversion recovery images as per our labs standard protocol.
Analysis of myocardial tagged images was performed using harmonic phase methodology (Myocardial Solutions, Morrisville, NC) as previously described to calculate circumferential strain (Ecc) at the base, mid, and apex and global Ecc.15 T1 maps, obtained prior to and after contrast administration as described by Messroghli et al,9 were used along with the subject’s hematocrit to calculate an extracellular volume map using manual registration in QMap from Medis. The was calculated as:
In cases where registration could not be adequately performed, native T1 and post-contrast T1 were traced separately and calculated manually. Regions of interest were manually drawn on T1 and maps within the LV mesocardium in the standard 16 segments, carefully avoiding partial volume averaging with blood-pool or epicardial fat. Areas of LGE were included as these areas were felt to be the most focal areas in a continuum of diffuse extracellular matrix expansion.10
Blood Biomarker Analysis
The Milliplex Map Human Cardiovascular Disease Panel 1 Magnetic Bead Kit (EMD Millipore Corporation, Billerica, MA. Cat # HCVD1MAG-67K) was used to detect plasma BNP, N-terminal pro-brain natriuretic peptide (NTproBNP), and troponin I according to manufacturer’s instructions. Seven working standards were generated by serial dilution (1:3) of the reconstituted standard provided in the kit. Two QC (Quality Control) samples were included in each plate run. Assay plate was read on Luminex 200 with XPONENT software using the parameters outlined in the assay kit instructions. The Milliplex Analyst 5.0 software was used for data analysis. The correlation efficiency for the Standard Curve was greater than 0.99 for each assay. All assays were run in duplicate, and the average coefficient of variation was less than 10%. Samples were excluded if the coefficient of variation was > 25%.
Statistical Analysis
Demographics and clinical findings at the subject’s first CMR are described as median and interquartile range (IQR) or N (%). Missing data pattern was investigated and displayed as diagram. Spearman correlations were used to evaluate the correlation between pertinent cardiac markers and indexed total QMT. Change from CMR 1 to CMR 2 and from CMR 1 to CMR 3 was reported as a median and IQR as well as mean ± standard deviation (SD); statistical significance was analyzed using a Wilcoxon signed-rank test. Univariate Cox proportional hazard regression modeling was performed with all-cause mortality as the primary outcome. Subjects were considered at risk from the first available visit that included a CMR until their time of death or censoring at last available followup. Schoenfeld residual trends over time were used to evaluate the proportional hazards assumption. Given the limited number of outcomes, univariate analysis was used to assess the association of potential confounders of interest (age, ambulatory status, positive pressure ventilation, current corticosteroids, current cardiac therapy) with mortality and to assess whether these confounders correlated with predictors of interest. Multivariable analysis was performed with predictors of interest and age, as well as any confounders with significant association with mortality.
RESULTS
A total of 78 subjects underwent 211 CMR studies between 2007–2020. Figure S1 demonstrates the missing data plot. The minimum number of CMR per subject was 1 (n=15) and there were 63 subjects with multiple CMR exams (maximum number 5). The median age of subjects at the time of the first CMR was 12.9 years (Table 1). Forty-six subjects (59%) were taking corticosteroids, with an additional 17 subjects having taken them previously (81% with current or prior corticosteroid use). Fifty-two subjects (74%) were on at least one cardiac medication at the time of the first CMR with angiotensin converting enzyme inhibitors (ACEi) being the most common. The majority of subjects (73%) were non-ambulatory at baseline visit.
Table 1:
Characteristics at first visit that included a cardiac MRI
| Duchenne Muscular Dystrophy Median (IQR*) or N (%) |
|
|---|---|
| Age (years) | 12.5 (10.3, 15.4) Range (7.4–27.5) |
| Height (cm) | 145 (127, 155) |
| Weight (kg) | 48.8 (34.7, 61.3) |
| Male gender | 100% |
| Current or prior corticosteroid use | 81% (N=63) |
| Current cardiac medications | |
| Angiotensin converting enzyme inhibitor | 62% (N=48) |
| Angiotensin receptor blocker | 9% (N=7) |
| Beta-Blocker | 31% (N=24) |
| Aldosterone inhibitor | 9% (N=7) |
| Ambulatory | 31% (N=24) |
| Positive pressure ventilation | 9% (N=7) |
| Mortality | 15 (19%) |
IQR – interquartile range
Cardiovascular findings at first CMR
The median LVEF at the time of first CMR was 57% with 32 subjects (41%) having an abnormal LVEF, defined as LVEF<55% (Table 2). DMD subjects did not have significant LV dilation. A total of 54 subjects had LGE (71%) with the majority at the base and mid-LV in the inferior and lateral segments. There was no significant correlation between indexed total QMT and LVEF, LV end diastolic and systolic volumes, global circumferential strain, or LGE FWHM (Table S1).
Table 2:
Cardiovascular data at first visit that included a cardiac MRI
| Duchenne Muscular Dystrophy Median (IQR*) or N (%) |
|
|---|---|
| Left ventricular (LV†) ejection fraction (%) | 57 (49, 60) |
| LV end diastolic volume indexed (ml/m2) | 64 (57, 78) |
| LV end systolic volume indexed (ml/m2) | 29 (23, 37) |
| LV cardiac output (ml/min) | 4.8 (3.8, 5.6) |
| LV cardiac index (ml/min/m2) | 3.5 (2.9, 4.1) |
|
| |
| Circumferential strain (Ecc‡) at base | −14.3 (−16.1, −12.6) |
| Ecc at mid-LV | −15.6 (−17.6, −13.1) |
| Global Ecc | −15.6 (−17.3, −13.3) |
|
| |
| Late gadolinium enhancement (LGE§) present | 71% (N=54) |
| Anterior Base | 14 (18%) |
| Anteroseptal Base | 9 (12%) |
| Inferoseptal Base | 10 (13%) |
| Inferior Base | 36 (46%) |
| Inferolateral Base | 44 (56%) |
| Anterolateral Base | 42 (54%) |
| Anterior Mid | 16 (21%) |
| Anteroseptal Mid | 8 (10%) |
| Inferoseptal Mid | 14 (18%) |
| Inferior Mid | 36 (46%) |
| Inferolateral Mid | 44 (56%) |
| Anterolateral Mid | 41 (53%) |
| Anterior Apex | 18 (23%) |
| Septal Apex | 18 (23%) |
| Inferior Apex | 21 (27%) |
| Lateral Apex | 25 (32%) |
| Apex | 17 (22%) |
| LGE global severity score | 2 (0, 3) |
| LGE full width half maximum (%) | 26.0 (0, 34.7) |
|
| |
| Native T1 mid (ms) | 1047 (1003, 1083) |
| T2 mid (ms) | 44.6 (42.8, 46.2) |
| Extracellular volume, mid (%) | 29.5 (25.0, 33.3) |
|
| |
| Troponin I (pg/ml) | 32.7 (32.7, 125) |
| Brain natriuretic peptide (pg/ml) | 34.3 (34.3, 34.3) |
| N-terminal pro b-type natriuretic peptide (pg/ml) | 80.1 (42.7, 169.8) |
IQR – interquartile range
LV – left ventricle
Ecc – circumferential strain
LGE – late gadolinium enhancement
Mortality and Follow Up
The median length of follow up after the first CMR was 5.0 years, IQR (3.1,7.0). Fifteen patients died during the course of follow up (19%), 8 as inpatients or in ambulance and the remainder at home. The median age of death was 17 years old. The deaths occurred a median of 4.9 years, IQR (3.6,6.2) from the first CMR and 3.4 years, IQR (1.3,4.0) from the final CMR, though all patients continued to have outpatient follow up that included echocardiograms to assess ventricular function. In 1 patient, details of death were unknown. In the remaining patients, 6 patients (40%) had primary cardiac mortality and 2 patients (13%) had cardiac mortality as the secondary cause. Seven patients (47%) had respiratory mortality as the primary cause. For 2 patients (18%), infection was either the primary or secondary cause. A scatterplot of baseline LVEF, age, and outcome is presented in Figure S2.
Biomarker change over time
The changes in CMR and blood biomarkers over time are detailed in Table 3. The median LVEF decreased significantly by 3% over a 1-year period and 5% over a 2-year period. The Ecc at the base and mid-LV also worsened (less negative) at 2-years by a median of 1.0% and 0.80%. Monitoring LGE change over time demonstrated 6 subjects increasing by 2 or 3 levels from years 1 to 3 while only 2 subjects had that level of progression from years 1 to 2. The LGE FWHM percent increased by a median of 5.9% in the first year and a median of 15.3% in the second year. These changes provide measurable CMR biomarkers now related to all-cause mortality in DMD.
Table 3:
Changes in cardiac magnetic resonance and blood biomarkers over time
| Cardiovascular Measure | N | Change in biomarkers from Visit 1 to Visit 2 | p-value | N | Change in biomarkers from Visit 1 to Visit 3 | p-value |
|---|---|---|---|---|---|---|
| Time between visits | Median 1.02 years IQR (0.98, 1.06) Range (0.84, 1.23) |
Median 2.03 years IQR (1.99, 2.09) Range (1.92, 2.53) |
||||
|
| ||||||
| Left ventricular (LV†) ejection fraction (%) | 47 | −3.0 (−4.5, −1.0) −2.7 ± 3.7 |
<0.001 | 37 | −5.0 (−7.0, −1.0) −4.6 ± 5.2 |
<0.001 |
| LV end diastolic volume indexed (ml/m2) | 47 | 0.0 (−5.0, 6.5) 1.2 ± 9.5 |
0.492 | 37 | 4.0 (−1.0, 11.0) 3.9 ± 11.6 |
0.03 |
| LV end systolic volume indexed (ml/m2) | 47 | 2.0 (−1.0, 7.0) 2.3 ± 6.0 |
0.013 | 37 | 5.0 (1.0, 9.0) 4.5 ± 6.5 |
<0.001 |
| LV cardiac index (L/min/m2) | 47 | −0.3 (−0.6, 0.1) 0.34 ± 0.9 |
0.005 | 37 | 0.0 (−0.5, 0.3) −0.16 ± 0.87 |
0.591 |
| Circumferential strain (Ecc‡) base (%) | 44 | 1.1 (−1.7, 3.0) 0.9 ± 3.1 |
0.074 | 34 | 1.0 (−1.4, 3.2) 0.9 ± 2.9 |
0.110 |
| Ecc mid-LV (%) | 45 | 0.2 (−1.7, 2.6) 0.6 ± 3.4 |
0.309 | 33 | 0.80 (−1.5, 2.8) 0.97 ± 2.9 |
0.151 |
| Global Ecc (%) | 44 | 0.9 (−1.8, 2.5) 0.6 ± 2.9 |
0.217 | 33 | 0.61 (−1.4, 2.8) 0.8 ± 3.6 |
0.126 |
|
| ||||||
| Late gadolinium enhancement (LGE) severity score | 47 | −1, 0.064 (n=3) 0, 0.596 (n=28) 1, 0.298 (n=14) 2, 0.021 (n=1) 3, 0.021 (n=1) |
0.003 | 34 | −1, 0.059 (n=2) 0, 0.412 (n=14) 1, 0.353 (n=12) 2, 0.088 (n=3) 3, 0.088 (n=3) |
<0.001 |
| Percent (LGE§) by full-width half maximum (%) | 37 | 5.9 (−4.6, 15.9) 7.2 ± 18.6 |
0.024 | 30 | 15.3 (3.7, 27.1) 14.8 ± 17.5 |
<0.001 |
| Native T1 mid (ms) | 43 | −11.0 (−48.5, 36.5) −16.1 ± 79.8 |
0.279 | 34 | −25.5 (−55.5, 23.8) −15.6 ± 68.5 |
0.106 |
| T2 mid (ms) | 42 | 1.75 (0.1, 3.3) 1.5 ± 2.6 |
<0.001 | 32 | 0.9 (0.08, 2.6) 1.5 ± 3.6 |
0.008 |
| Extracellular volume, mid (%) | 43 | 0.0 (−3.0, 3.2) 0.2 ± 5.2 |
0.988 | 31 | −0.7 (−4.0, 4.5) 0.00 ± 4.9 |
0.875 |
|
| ||||||
| Brain natriuretic peptide (pg/ml) | 37 | −16.0 (−16.0, 0.0) −16.3 ± 41.6 |
0.014 | 26 | −16.0 (−16.0, 0.0) −15.8 ± 39.4 |
0.014 |
| N-terminal pro b-type natriuretic peptide (pg/ml) | 37 | 36.1 (−39.6, 94.6) 31.1 ± 174.3 |
0.065 | 26 | 41.8 (2.0, 172.8) 64.1 ± 193.1 |
0.037 |
| Troponin I (pg/ml) | 34 | 0.0 (−37.5, 63.7) 132.1 ± 871.9 |
0.425 | 25 | 23.0 (−38.7, 137.2) −10.1 ± 326.8 |
0.286 |
Reported as median (interquartile range) and mean ± standard deviation
IQR – interquartile range
LV – left ventricle
Ecc – circumferential strain
LGE – late gadolinium enhancement
Modeling of All-Cause Mortality
Modeling for all-cause mortality based on the first available CMR is reported in Table 4. There was no evidence that the proportional hazard assumption was violated for any of the models. Notably, LVEF had a strong association with mortality, with a hazard ratio of 1.32 for each 3% decrease in LVEF. Global and mid-LV circumferential strain also demonstrated significant associations with mortality, with 1.5% and 1.1% increases (less negative or worsening) in strain leading to increased mortality (HR 1.33 for both). In terms of volumetrics, increases in indexed LV end diastolic and systolic volumes by 1.5ml/m2 and 3ml/m2 both associated with mortality (HR 1.07 and 1.19, respectively). Increased LGE FWHM (12%) associated with mortality (HR 1.56) while increased native T1 (20ms) in the mid-LV was protective (HR 0.90). NTproBNP was the only blood biomarker that associated with mortality. Association curves for LVEF, LGE FWHM, global Ecc, and NTproBNP and probability of 5-year mortality are shown in Figure 1. There was no association between confounders of interest and all-cause mortality (Table S2); age was included as a covariate in a multivariable model and this did not alter the univariate associations (Table S3).
Table 4:
Model for all-cause mortality based on first available visit that included a cardiac MRI
| Measure | N | N_e* | Hazard Ratio Confidence interval |
P-value |
|---|---|---|---|---|
| Left ventricular (LV†) ejection fraction per 3% decrease | 78 | 15 | 1.32 1.12–1.55 |
<0.001 |
| LV end diastolic volume indexed per 4ml/m2 increase | 77 | 14 | 1.20 1.09–1.32 |
<0.001 |
| LV end systolic volume indexed per 2ml/m2 increase | 77 | 14 | 1.12 1.06–1.19 |
<0.001 |
| LV cardiac index per 1L/min/m2 increase | 76 | 13 | 1.53 0.80–2.92 |
0.200 |
|
| ||||
| Circumferential strain (Ecc‡) at base per 1% increase (less negative) | 73 | 12 | 1.20 0.98–1.46 |
0.072 |
| Ecc at mid-LV per 0.8% increase (less negative) | 73 | 12 | 1.23 1.06–1.43 |
0.007 |
| Global Ecc per 0.6% increase (less negative) | 73 | 12 | 1.12 1.00–1.25 |
0.043 |
|
| ||||
| Presence of late gadolinium enhancement (LGE§) | 76 | 15 | 1.19 0.33–4.32 |
0.800 |
| LGE global severity score per 1 unit increase | 73 | 15 | 1.34 0.86–2.10 |
0.190 |
| LGE full width half maximum per 5.9% increase | 74 | 15 | 1.24 1.06–1.46 |
0.008 |
|
| ||||
| Native T1 mid per 11ms decrease | 71 | 10 | 1.18 1.04–1.32 |
0.008 |
| T2 mid per 1ms increase | 66 | 8 | 1.09 0.84–1.42 |
0.520 |
| Extracellular volume, mid-LV per 1% increase | 65 | 7 | 1.04 0.95–1.15 |
0.400 |
|
| ||||
| Brain natriuretic peptide per 1pg/ml increase | 60 | 11 | 1.00 0.99–1.01 |
0.500 |
| N-terminal pro b-type natriuretic peptide per 50 pg/ml increase | 60 | 11 | 1.18 1.00–1.39 |
0.045 |
| Troponin I per 1pg/ml increase | 60 | 11 | 1.00 0.99–1.00 |
0.390 |
N_e – number of events
LV – left ventricle
Ecc – circumferential strain
LGE – late gadolinium enhancement
Figure 1:
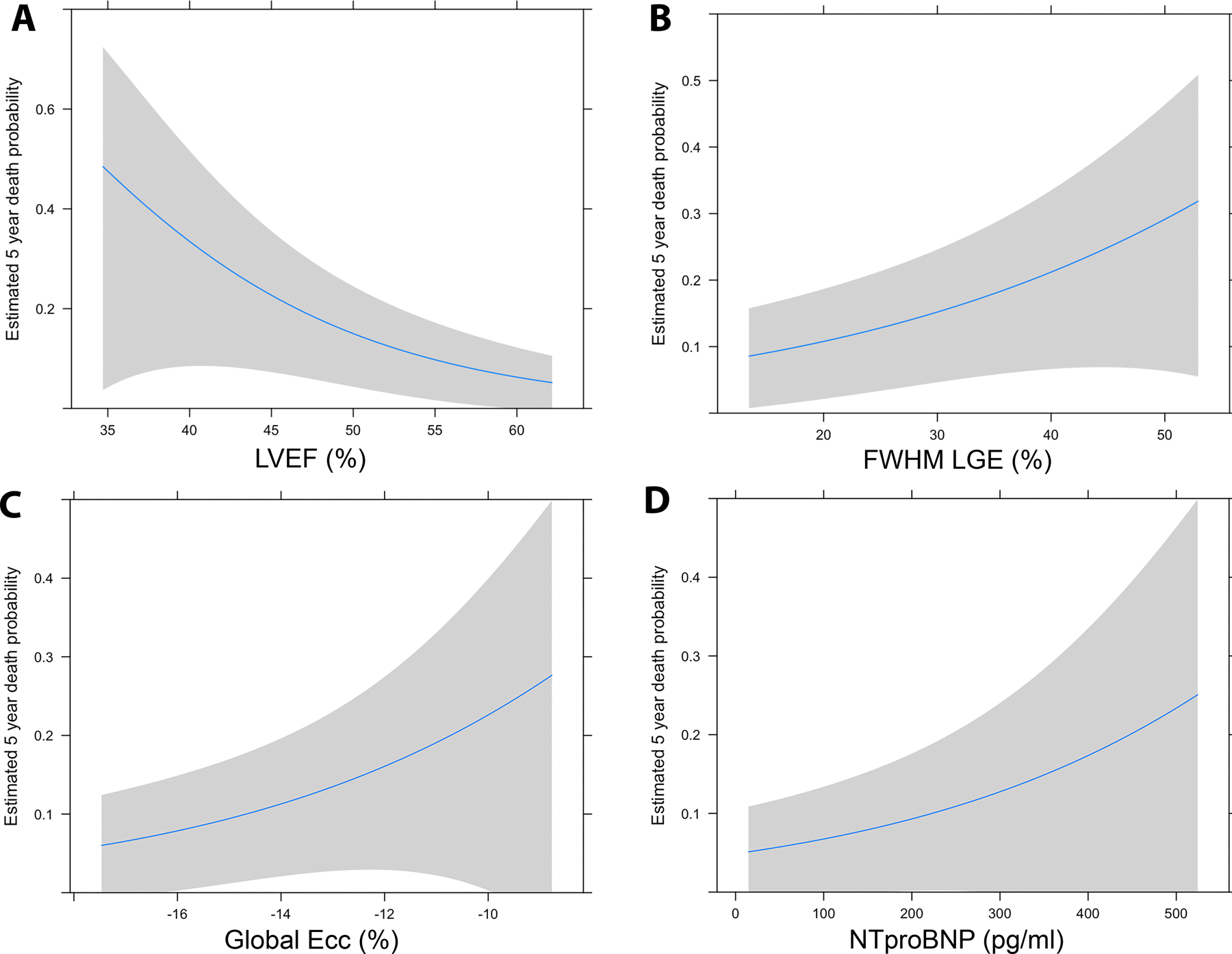
Estimated 5-year mortality based on left ventricular ejection fraction (LVEF) (A), Late gadolinium enhancement (LGE) measured with full-width half maximum technique (FWHM) (B), LV global myocardial strain measured from tagged images (Ecc global) (C), and n-terminal pro brain natriuretic peptide (NTpro-BNP) (D).
DISCUSSION
We believe this is the first report to evaluate comprehensive CMR and blood biomarkers as surrogate outcome measures of all-cause mortality in a large cohort of DMD patients. This report also provides the change over time for pertinent CMR and blood biomarkers. These novel findings suggest that LVEF, indexed LV end diastolic and systolic volumes, Ecc, LGE FWHM, and NTproBNP have the strongest association with mortality and should be considered for outcome measures in therapeutic trials, either individually or as components of a composite end point. Combining the progression data with mortality data provides insight into the potential use of these measures in therapeutic DMD trials. Specifically, a decrease in LVEF of 3%, which is the median decrease in LVEF over a 2-year period, has a hazard ratio of 1.32 in its association with mortality. Therefore, a medication that could stop this progression would be expected to have a similar beneficial effect for survival. Similarly, the median progression of global Ecc over 1-year is 1.5% and the median progression of FWHM LGE over a 2-year period is 12%, and these levels of progression have hazard ratios of 1.33 and 1.56. If medications are able to reverse progression, they would be expected to have an even larger effect on survival.
The rates of change and association with mortality presented here are critical for designing therapeutic trials. The LVEF decline is similar to that of prior single and multicenter studies, suggesting that the cohort is generalizable.16–18 Most trials will be 1 or 2 years in duration and a comprehensive understanding of the change in biomarkers over time is necessary to adequately power a trial. Therefore, our data focuses on 1- and 2-year progression in DMD. More importantly, trials must select the optimal outcome measures and these outcome measures must be accepted by both the scientific community and the FDA (assuming an official FDA indication is desired). In order to achieve this acceptance, an association with mortality is crucial. This study is one of the largest DMD cohorts of which we are aware that includes longitudinal comprehensive CMR and blood sample phenotyping in prospectively enrolled subjects.
Currently, the relationship between cardiac biomarkers and mortality is unclear. There have only been a few small, retrospective studies, most of which evaluated differences between groups and did not perform survival analyses. Multiple studies have demonstrated that echocardiographic measures of function, such as fractional shortening, ejection fraction, and chamber size, primarily end diastolic ventricular diameter, associate with cardiac mortality.19–23 Of note, DMD echocardiographic images tend to be poor with high inter- and intra-observer variability, particularly in older boys, and CMR has replaced echocardiography as the modality of choice for both clinical and research assessment.24, 25
There are even fewer studies evaluating CMR findings and mortality. In a small cohort, Menon demonstrated that the LGE severity score, LVEF, and indexed LV end systolic volume correlated with mortality.26 Florian also demonstrated that presence of transmural LGE and depressed LVEF were poor prognostic indicators in DMD and Becker muscular dystrophy, though the majority of their outcomes were adverse events (N=22) and not mortality (N=2). In addition, only a minority of their subjects had DMD (N=20), and the Becker subjects (N=68) had larger LV volumes than DMD, calling into question whether these diagnoses should be grouped together.27 Wittlieb-Weber demonstrated that DMD subjects who had died had lower CMR LVEF but no difference in LGE when compared to those still living, but only a small subset of subjects in their study underwent CMR.22 Our results address many of the limitations of prior studies in that we present a larger DMD cohort with longitudinal comprehensive phenotyping using CMR, the standard of care for measurement of function in DMD. Our data also supports a role for LGE in the progression of DMD cardiomyopathy and mortality. The number of patients who demonstrated an increase in LGE score of +2 or 3 was 4-fold greater over a 2-year period. This correlated with a 3-fold increase in LGE FWHM. Both of these findings provide quantifiable trial outcome measures for LGE in DMD.
One of the interesting findings of this study is the association between shorter native T1 times at the mid-LV and mortality. While native T1 has classically been felt to increase with fibrosis, and therefore with DMD disease progression, recent analysis suggests that native T1 decreases with progression of DMD cardiovascular disease.28 This is likely due to the replacement of myocytes with fat. Future analyses should clarify whether fatty replacement itself associates with mortality.
While few outcome studies exist linking CMR biomarkers to mortality, multiple studies have used CMR biomarkers to evaluate therapeutic effects in DMD. Studies evaluating the effects of aldosterone inhibition in DMD used both CMR LVEF and Ecc as outcome measures.17, 29, 30 More recently, Ecc has been shown to associate with the development of LV dysfunction.31 Our data validate the prior use of LVEF and Ecc as outcome measures in therapeutic trials. Our data show an increase in global Ecc of 1.5% over a 1-year period, which is relatively similar to the 2.2% increase seen in the eplerenone study.17
The utility of standard cardiac blood biomarkers has also been unclear in DMD. Some studies suggest either no correlation or a weak correlation between biomarkers, while others demonstrate stronger correlations.32–35 However, evaluations of cardiac biomarkers and mortality have been less common. Cheeran demonstrated higher NTproBNP values in non-survivors compared with survivors, and Wittlieb-Weber demonstrated a similar finding for both NTproBNP and BNP levels, though neither performed a survival analysis.22, 36 In this study, NTproBNP associated with mortality, while BNP and TNI did not.
The optimal timing for obtaining CMR and blood biomarkers remains unclear. It is certainly possible to obtain the biomarkers too early, well before any myocardial changes have manifested. Whether biomarkers can be obtained too late is an interesting question – perhaps biomarkers that are early markers of disease, such as the presence of LGE, have a decreased association with mortality later in the disease process. To evaluate this question, we also obtained mortality associations for the most recent available CMR for all subjects in the cohort (Table S4). Interestingly, volumetric and functional biomarkers, such as LVEF, indexed LV end diastolic and systolic volumes, and LV strain, remained significantly associated with mortality, as did NTproBNP. However, none of the tissue characterization biomarkers associated with mortality, including native T1 and LGE FWHM.
Limitations
This study is a single center study with all of the associated limitations. However, this cohort was extensively and prospectively phenotyped from a cardiovascular perspective, providing significant advantages over retrospective studies. The number of deaths in this cohort did not allow for multivariable analysis. In addition, multiple variables were evaluated in order to better identify the best biomarkers for future study. While these biomarkers are identified as promising, there is certainly multicollinearity with some (particularly the volumetric and functional measures) and the optimal biomarker or combination of biomarkers cannot be determined. However, all of the significant biomarkers in this study represent clinically utilized and promising candidates for further evaluation and validation. Of note, there are multiple assays available to measure BNP, NTproBNP, and TNI and these data only apply to the specific assays used in this analysis. An assessment of cardiovascular mortality, rather than all-cause mortality, may reveal stronger associations with predictors of interest, but the relatively small number of purely cardiovascular deaths did not allow for a subset analysis in this cohort. However, all-cause mortality is certainly a clinically significant outcome measure. Due to the timing of research on aldosterone inhibition, many of the patients in this study were not yet on eplerenone or spironolactone at the time of first CMR, though 35 (45%) had started it by the conclusion of the study. Finally, this study included standardized imaging with the majority of patients enrolled prospectively. While this is a significant advantage for image analysis, the generalizability to other centers with different CMR protocols is unclear.
CONCLUSIONS
Identification of potentially modifiable outcome measures of cardiovascular disease in DMD is critical for the development of cardiovascular therapeutics. This is the first study to prospectively assess the relationship of comprehensive CMR and blood biomarkers to mortality. Our data demonstrate LVEF, indexed LV end diastolic and systolic volumes, LGE FWHM, Ecc, and NTproBNP are associated with mortality and serve as the best outcome measures for cardiovascular therapeutic trials.
Supplementary Material
CLINICAL PERSPECTIVES :
What is new?
In patients with Duchenne muscular dystrophy (DMD), functional measures including left ventricular ejection fraction, left ventricular end diastolic and systolic volumes, and circumferential myocardial strain associate with all-cause mortality.
Percent late gadolinium enhancement (LGE) measured using the full width half maximum technique and native T1 mapping are the only methods of tissue characterization that associate with all-cause mortality.
N-terminal pro brain natriuretic peptide (NTproBNP) associates with all-cause mortality, but BNP and troponin do not.
What are the clinical implications?
DMD cardiovascular medications need to be started early in order to change the course of disease, in most cases years before death. Therefore, surrogate outcome measures are necessary to evaluate novel therapies.
These results demonstrate the association between CMR and blood biomarkers and all-cause mortality in DMD and report the changes in these biomarkers over time.
This manuscript provides a comprehensive, prospective assessment of potential surrogate outcome measures for clinical trial development in DMD.
ACKNOWLEDGMENTS:
None.
Sources of Funding:
Research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number K23HL123938 and R56HL141248 (Bethesda, MD) (Soslow) and K08HL155852 (Raucci). The project was supported by the National Center for Research Resources, Grant UL1 RR024975–01, and is now at the National Center for Advancing Translational Sciences, Grant 2 UL1 TR000445–06 (Bethesda, MD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This project was supported by the Food and Drug Administration Orphan Products Grant R01FD006649 (Soslow).
This project was supported by the Fighting Duchenne Foundation and the Fight DMD/Jonah & Emory Discovery Grant (Nashville, TN) (Soslow).
The sponsors and funders had no role in the design and conduct of the study or in the collection, analysis, and interpretation of the data, or in the preparation, review, or approval of the manuscript.
Non-Standard Abbreviations and Acronyms:
- BNP
brain natriuretic peptide
- CMR
cardiac magnetic resonance
- DMD
Duchenne muscular dystrophy
- Ecc
circumferential strian
extracellular volume
- FWHM
full width half maximum
- LGE
late gadolinium enhancement
- LV
left ventricle
- LVEF
left ventricular ejection fraction
- NTproBNP
N-terminal pro b-type natriuretic peptide
Footnotes
DISCLOSURES:
Dr. Soslow is on the Advisory Board for PepGen. Drs. Soslow and Hor are consultants for Sarepta.
References
- 1.Kieny P, Chollet S, Delalande P, Le Fort M, Magot A, Pereon Y and Perrouin Verbe B. Evolution of life expectancy of patients with Duchenne muscular dystrophy at AFM Yolaine de Kepper centre between 1981 and 2011. Ann Phys Rehabil Med 2013;56:443–54. [DOI] [PubMed] [Google Scholar]
- 2.McNally EM, Kaltman JR, Benson DW, Canter CE, Cripe LH, Duan D, Finder JD, Groh WJ, Hoffman EP, Judge DP, et al. Contemporary cardiac issues in Duchenne muscular dystrophy. Working Group of the National Heart, Lung, and Blood Institute in collaboration with Parent Project Muscular Dystrophy. Circulation 2015;131:1590–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bourke JP, Watson G, Spinty S, Bryant A, Roper H, Chadwick T, Wood R, McColl E, Bushby K, Muntoni F, et al. Preventing Cardiomyopathy in DMD: A Randomized Placebo-Controlled Drug Trial. Neurol Clin Pract 2021;11:e661–e668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Food and Drug Administration Center for Evaluation and Research. Duchenne Muscular Dystrophy and Related Dystrophinopathies: Developing Drugs for Treatment Guidance for Industry. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/duchenne-muscular-dystrophy-and-related-dystrophinopathies-developing-drugs-treatment-guidance. 2018. [Google Scholar]
- 5.Posner AD, Soslow JH, Burnette WB, Bian A, Shintani A, Sawyer DB and Markham LW. The correlation of skeletal and cardiac muscle dysfunction in Duchenne muscular dystrophy. Journal of Neuromuscular Disorders 2016;3:91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soslow JH, Damon SM, Crum K, Lawson MA, Slaughter JC, Xu M, Arai AE, Sawyer DB, Parra DA, Damon BM, et al. Increased myocardial native T1 and extracellular volume in patients with Duchenne muscular dystrophy. J Cardiovasc Magn Reson 2016;18:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soslow JH, Damon BM, Saville BR, Lu Z, Burnette WB, Lawson MA, Parra DA, Sawyer DB and Markham LW. Evaluation of post-contrast myocardial t1 in duchenne muscular dystrophy using cardiac magnetic resonance imaging. Pediatr Cardiol 2015;36:49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soslow JH, Xu M, Slaughter JC, Crum K, Chew JD, Burnette WB, Su YR, Tomasek K, Parra DA and Markham LW. The Role of Matrix Metalloproteinases and Tissue Inhibitors of Metalloproteinases in Duchenne Muscular Dystrophy Cardiomyopathy. Journal of cardiac failure 2019;25:259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Messroghli DR, Radjenovic A, Kozerke S, Higgins DM, Sivananthan MU and Ridgway JP. Modified Look-Locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart. Magn Reson Med 2004;52:141–6. [DOI] [PubMed] [Google Scholar]
- 10.Moon JC, Messroghli DR, Kellman P, Piechnik SK, Robson MD, Ugander M, Gatehouse PD, Arai AE, Friedrich MG, Neubauer S, et al. Myocardial T1 mapping and extracellular volume quantification: a Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J Cardiovasc Magn Reson 2013;15:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kellman P and Hansen MS. T1-mapping in the heart: accuracy and precision. J Cardiovasc Magn Reson 2014;16:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xue H, Shah S, Greiser A, Guetter C, Littmann A, Jolly MP, Arai AE, Zuehlsdorff S, Guehring J and Kellman P. Motion correction for myocardial T1 mapping using image registration with synthetic image estimation. Magn Reson Med 2012;67:1644–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T and Verani MS. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. J Nucl Cardiol 2002;9:240–5. [DOI] [PubMed] [Google Scholar]
- 14.Raucci FJ Jr., Xu M, George-Durrett K, Crum K, Slaughter JC, Parra DA, Markham LW and Soslow JH. Non-contrast cardiovascular magnetic resonance detection of myocardial fibrosis in Duchenne muscular dystrophy. J Cardiovasc Magn Reson 2021;23:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simpson SA, Field SL, Xu M, Saville BR, Parra DA and Soslow JH. Effect of Weight Extremes on Ventricular Volumes and Myocardial Strain in Repaired Tetralogy of Fallot as Measured by CMR. Pediatr Cardiol 2017;39:575–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tandon A, Villa CR, Hor KN, Jefferies JL, Gao Z, Towbin JA, Wong BL, Mazur W, Fleck RJ, Sticka JJ, et al. Myocardial fibrosis burden predicts left ventricular ejection fraction and is associated with age and steroid treatment duration in duchenne muscular dystrophy. J Am Heart Assoc 2015;4:e001338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raman SV, Hor KN, Mazur W, Halnon NJ, Kissel JT, He X, Tran T, Smart S, McCarthy B, Taylor MD, et al. Eplerenone for early cardiomyopathy in Duchenne muscular dystrophy: a randomised, double-blind, placebo-controlled trial. Lancet neurology 2015;14:153–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Batra A, Barnard AM, Lott DJ, Willcocks RJ, Forbes SC, Chakraborty S, Daniels MJ, Arbogast J, Triplett W, Henricson EK, et al. Longitudinal changes in cardiac function in Duchenne muscular dystrophy population as measured by magnetic resonance imaging. BMC Cardiovasc Disord 2022;22:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corrado G, Lissoni A, Beretta S, Terenghi L, Tadeo G, Foglia-Manzillo G, Tagliagambe LM, Spata M and Santarone M. Prognostic value of electrocardiograms, ventricular late potentials, ventricular arrhythmias, and left ventricular systolic dysfunction in patients with Duchenne muscular dystrophy. Am J Cardiol 2002;89:838–41. [DOI] [PubMed] [Google Scholar]
- 20.Segawa K, Sugawara N, Maruo K, Kimura K, Komaki H, Takahashi Y and Sasaki M. Left Ventricular End-Diastolic Diameter and Cardiac Mortality in Duchenne Muscular Dystrophy. Neuropsychiatr Dis Treat 2020;16:171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang M, Birnkrant DJ, Super DM, Jacobs IB and Bahler RC. Progressive left ventricular dysfunction and long-term outcomes in patients with Duchenne muscular dystrophy receiving cardiopulmonary therapies. Open Heart 2018;5:e000783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wittlieb-Weber CA, Knecht KR, Villa CR, Cunningham C, Conway J, Bock MJ, Gambetta KE, Lal AK, Schumacher KR, Law SP, et al. Risk Factors for Cardiac and Non-cardiac Causes of Death in Males with Duchenne Muscular Dystrophy. Pediatr Cardiol 2020;41:764–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schram G, Fournier A, Leduc H, Dahdah N, Therien J, Vanasse M and Khairy P. All-cause mortality and cardiovascular outcomes with prophylactic steroid therapy in Duchenne muscular dystrophy. J Am Coll Cardiol 2013;61:948–54. [DOI] [PubMed] [Google Scholar]
- 24.Brunklaus A, Parish E, Muntoni F, Scuplak S, Tucker SK, Fenton M, Hughes ML and Manzur AY. The value of cardiac MRI versus echocardiography in the pre-operative assessment of patients with Duchenne muscular dystrophy. Eur J Paediatr Neurol 2015;19:395–401. [DOI] [PubMed] [Google Scholar]
- 25.Soslow JH, Xu M, Slaughter JC, Stanley M, Crum K, Markham LW and Parra DA. Evaluation of Echocardiographic Measures of Left Ventricular Function in Patients with Duchenne Muscular Dystrophy: Assessment of Reproducibility and Comparison to Cardiac Magnetic Resonance Imaging. J Am Soc Echocardiogr 2016;29:983–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Menon SC, Etheridge SP, Liesemer KN, Williams RV, Bardsley T, Heywood MC and Puchalski MD. Predictive value of myocardial delayed enhancement in Duchenne muscular dystrophy. Pediatr Cardiol 2014;35:1279–85. [DOI] [PubMed] [Google Scholar]
- 27.Florian A, Ludwig A, Engelen M, Waltenberger J, Rosch S, Sechtem U and Yilmaz A. Left ventricular systolic function and the pattern of late-gadolinium-enhancement independently and additively predict adverse cardiac events in muscular dystrophy patients. J Cardiovasc Magn Reson 2014;16:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lang SM, Alsaied T, Khoury PR, Ryan TD and Taylor MD. Variations in native T1 values in patients with Duchenne muscular dystrophy with and without late gadolinium enhancement. Int J Cardiovasc Imaging 2020;37:635–642. [DOI] [PubMed] [Google Scholar]
- 29.Raman SV, Hor KN, Mazur W, He X, Kissel JT, Smart S, McCarthy B, Roble SL and Cripe LH. Eplerenone for early cardiomyopathy in Duchenne muscular dystrophy: results of a two-year open-label extension trial. Orphanet J Rare Dis 2017;12:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raman SV, Hor KN, Mazur W, Cardona A, He X, Halnon N, Markham L, Soslow JH, Puchalski MD, Auerbach SR, et al. Stabilization of Early Duchenne Cardiomyopathy With Aldosterone Inhibition: Results of the Multicenter AIDMD Trial. J Am Heart Assoc 2019;8:e013501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siddiqui S, Alsaied T, Henson SE, Gandhi J, Patel P, Khoury P, Villa C, Ryan TD, Wittekind SG, Lang SM, et al. Left Ventricular Magnetic Resonance Imaging Strain Predicts the Onset of Duchenne Muscular Dystrophy-Associated Cardiomyopathy. Circ Cardiovasc Imaging 2020;13:e011526. [DOI] [PubMed] [Google Scholar]
- 32.Mohyuddin T, Jacobs IB and Bahler RC. B-type natriuretic peptide and cardiac dysfunction in Duchenne muscular dystrophy. International journal of cardiology 2007;119:389–91. [DOI] [PubMed] [Google Scholar]
- 33.Matsumura T, Saito T, Fujimura H and Shinno S. Cardiac troponin I for accurate evaluation of cardiac status in myopathic patients. Brain Dev 2007;29:496–501. [DOI] [PubMed] [Google Scholar]
- 34.Schade van Westrum SM, Hoogerwaard EM, Dekker L, Standaar TS, Bakker E, Ippel PF, Oosterwijk JC, Majoor-Krakauer DF, van Essen AJ, Leschot NJ, et al. Cardiac abnormalities in a follow-up study on carriers of Duchenne and Becker muscular dystrophy. Neurology 2011;77:62–6. [DOI] [PubMed] [Google Scholar]
- 35.Demachi J, Kagaya Y, Watanabe J, Sakuma M, Ikeda J, Kakuta Y, Motoyoshi I, Kohnosu T, Sakuma H, Shimazaki S, et al. Characteristics of the increase in plasma brain natriuretic peptide level in left ventricular systolic dysfunction, associated with muscular dystrophy in comparison with idiopathic dilated cardiomyopathy. Neuromuscul Disord 2004;14:732–9. [DOI] [PubMed] [Google Scholar]
- 36.Cheeran D, Khan S, Khera R, Bhatt A, Garg S, Grodin JL, Morlend R, Araj FG, Amin AA, Thibodeau JT, et al. Predictors of Death in Adults With Duchenne Muscular Dystrophy-Associated Cardiomyopathy. J Am Heart Assoc 2017;6:e006340. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


