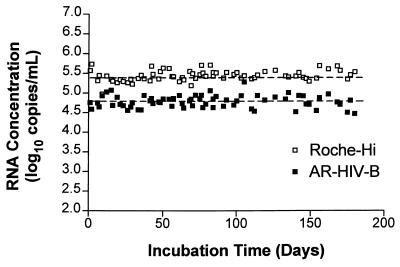
FIG. 6.
Comparison of Armored RNA positive control and the HIV-1 Monitor assay high-positive control (Roche-Hi) used in a clinical setting over 180 days. AR–HIV-B was added to clarified EDTA-anticoagulated plasma to a final concentration of ∼65,000 copies/ml, aliquoted into 0.2-ml samples, and stored at −20°C until it was used in the HIV-1 Monitor assay to determine the RNA copy number. The Armored RNA positive control and the HIV-1 Monitor assay high-positive control were used in clinical runs two to four times per week for 180 days. For the Armored RNA standard, the mean was 64,598 copies/ml, the range was 31,760 to 191,716 copies/ml (4.50 to 5.28 log10), and the coefficient of variation was 40%. For the HIV-1 Monitor assay high-positive control, the mean was 290,537 copies/ml; the range was 94,345 to 544,737 copies/ml (4.97 to 5.74 log10), and the coefficient of variation was 32%. The dashed lines represent the means for the two different positive controls.