Figure 4: Overview of different components of AI/ML-enabled software as a medical device in premarket submissions.
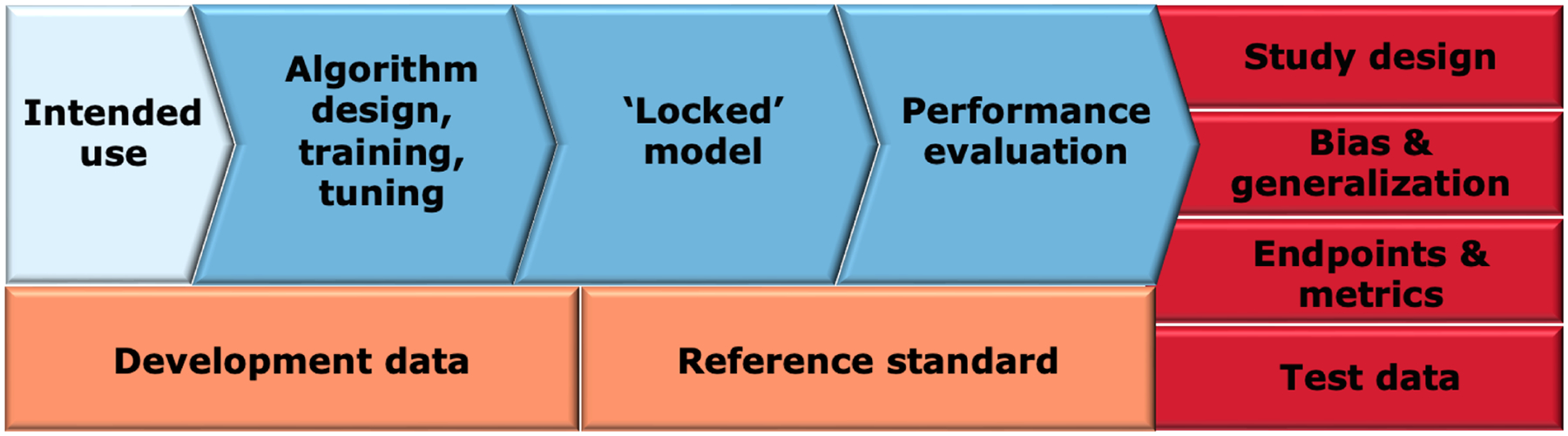
The intended use of the device (light blue) influences the data used for device development and the associated reference standard (orange). The device development process (blue) includes the algorithm development, a resulting locked model, and model evaluation. Clinical performance evaluation (red) consists of several interrelated components such as study design, bias and generalization assessment, selection of appropriate endpoints and metrics and selection of testing data that is representative of the target population.
