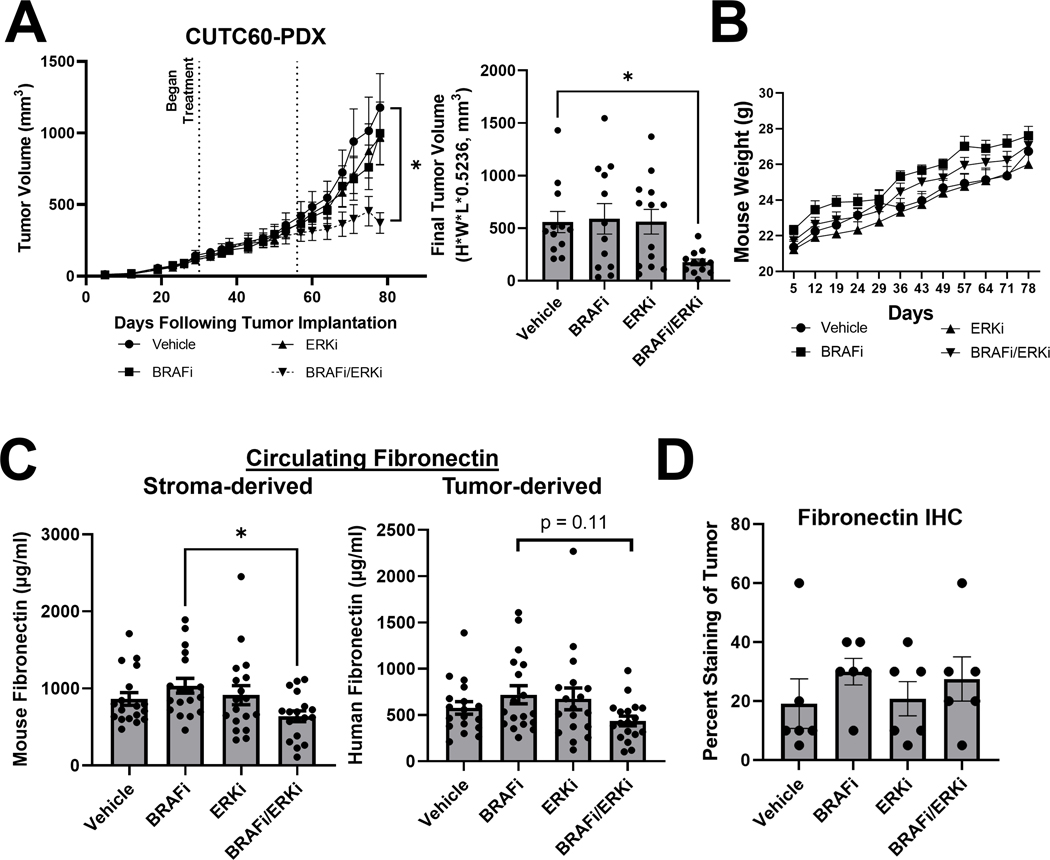
Figure 5. Combined BRAF and ERK1/2 inhibition reduces tumor growth and fibronectin in vivo.
A) Patient-derived xenograft (PDX) tumor chunks were injected into the flanks of athymic nude mice, allowed to grow to an average of 100mm3, at which point treatment with vehicle, BRAFi, ERKi, or the combination was performed via oral gavage. After 30 days of treatment (day 55 post-injection), doses of BRAFi and ERKi were increased. B) Mice were weighed weekly. C) At the time of sacrifice, plasma was isolated from blood samples. Mouse-specific and human-specific FN1 ELISA assays (Abcam) were performed to distinguish between stroma-derived and tumor-derived circulating fibronectin, respectively. D) Immunohistochemistry was performed on tumor sections for FN1. Results displayed as mean +/− SEM. *, p<0.05. BRAFi: dabrafenib (30 mg/kg prior to day 55, 50 mg/kg post), ERKi: ulixertinib (50 mg/kg prior to day 55, 100 mg/kg post), FN1: fibronectin.