Figure 1. Participant flow throughout the study.
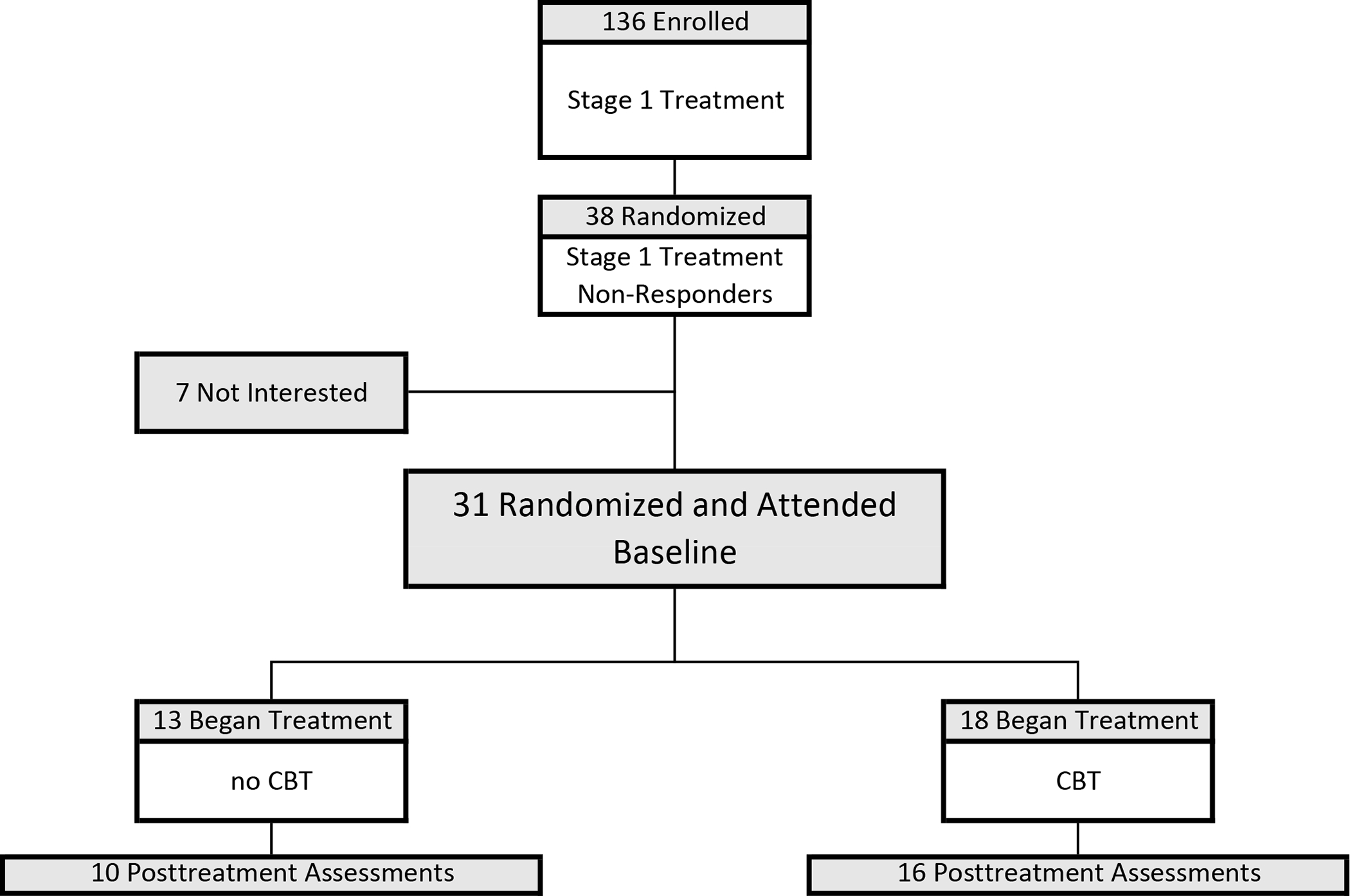
Participant flow through this randomized controlled trial (RCT) testing CBT versus no-CBT in addition to continuing double-blinded pharmacotherapy from Stage 1 initial acute trial (either naltrexone/bupropion (NB) or placebo) for patients with binge-eating disorder who were non-responders to acute treatments (Stage 1). Stage 1 treatment (N=136) was a RCT testing naltrexone/bupropion and behavioral weight loss (BWL), alone and together, using a 2×2 balanced factorial design, described previously (19). Of the 136 participants receiving Stage 1 treatments, 38 were categorized as treatment non-responders and were eligible for this Stage 2 trial for non-responders. “Randomization” (N=38) was the point at which the continuing (double-blinded) medication was ordered from the pharmacy once a participant was categorized as a “non-responder.” Participants were then scheduled for their “baseline session” at which time they were informed of their randomization (CBT or no-CBT) plus instructed to continue their medication, which was immediately available. Of the 38 randomized, 7 were excluded because of not attending baseline session or “not interested.” For this Stage 2 trial, the “intention-to-treat” sample (N=31) was defined as randomized plus attended the baseline session.
Of the N=31 randomized plus attended baseline session in this trial, 18 received cognitive-behavioral therapy (CBT) and 13 did not receive cognitive-behavioral therapy (no-CBT). Posttreatment assessments were obtained for 88.9% (N=16/18) of those receiving CBT and for 76.9% (N=10/13) of those in the no-CBT condition. Table 1 summarizes the Stage 1 treatments (a stratifying variable in the randomization schedule) received by the participants in the CBT and no-CBT conditions. See Supplemental Figure 1 for additional detailed description of the history of Stage 1 treatments for the participants at each step of this Stage 2 study.
