Abstract
Purpose:
Bacterial infection following spinal fusion is a major clinical concern with up to 20% incidence. An ultrasound-triggered bulk release system to combat post-surgical bacterial survival was designed and evaluated.
Methods:
Polylactic acid (PLA) clips were loaded with vancomycin (VAN) and microbubbles (Sonazoid, GE HealthCare) in vitro. Stability was determined over 14 days. VAN-loaded clips were submerged in water and insonated using a Logiq E10 scanner (GE HealthCare) with a curvilinear C6 probe. Doppler-induced VAN release was quantified using spectrophotometry. For in vivo testing, clips were loaded with methylene blue (MeB) solution and Sonazoid. These clips were implanted into a rabbit along the spine at L2 and L5, as well as a pig at L1 and L3, then insonated in Doppler mode using the C6 probe.
Results:
Sonazoid microbubbles were better preserved when incubated in VAN compared to distilled water at 4°C, 25°C, and 37°C incubation temperatures (p = 0.0131). Contrast enhancement was observed from both solutions when incubated at 4°C storage conditions. Insonated clips achieved average cumulative VAN release of 101.8 ± 2.8% (81.4 ± 2.8mg) after 72 hours. Uninsonated clips had only 0.3 ± 0.1% (0.3 ± 0.1mg) average cumulative VAN release (p < 0.0001). Clips retrieved from the rabbit did not rupture with insonation nor produce MeB staining of surrounding tissues. In the pig, the PLA film was visibly ruptured and MeB tissue was observed following insonation, whereas the uninsonated clip was intact.
Conclusion:
These results demonstrate ultrasound-triggered release of an encapsulated prophylactic solution and provide an important proof-of-concept for continuing large animal evaluations for translational merit.
Keywords: spine, infection, prophylaxis, ultrasound triggered release
Introduction
Patients who have failed conservative treatment, such as physical therapy, epidural injection, and minimally invasive discectomy or laminectomy, but are still experiencing intractable regional back pain and radiculopathy, become candidates for spinal fusion surgery [1]. The goal of spinal fusion is to reduce back pain by applying a bone graft between two or more unstable vertebrae, and usually involves implantation of instrumentation, also known as spinal hardware, to improve the rate of bone fusion [1]. In the United States, elective spinal fusion cases have increased by over 275% over the past 2 decades [2], with an estimated 400,000 cases performed annually as of 2014 [3]. However, bacterial infection remains a major clinical concern of these operations, with the incidence of post-surgical infections as high as 20% despite aggressive peri-operative antibiotic regimes [4, 5]. Other patient factors, such as age, obesity, immune compromise, and overall health, contribute to this infection risk [5].
The approach and duration of the operation are also factors that are directly related to infection risk [6]. To mitigate these factors, surgeons adhere to multiple procedures to minimize bacterial contamination, with standard of care application of intravenous antibiotics prior to the operation [7]. To further decrease infection risk, many surgeons place approximately 1-2 g of powdered vancomycin (VAN) into the dead space (~1-2 cm3) surrounding the implant during wound closure [7, 8]. While these measures have markedly reduced infection rates, infections still occur. The most common microorganism responsible for spinal infections is Staphylococcus aureus, where Staphylococci account for approximately 80% of all spinal infections [9-12]. The remaining 20% of spinal infections are attributed to Streptococci, Enterococci, and anerobic microorganisms [10-12].
The presence of the spinal instrumentation presents a fertile surface for pathogen colonization and biofilm formation. According to the NIH, over 80% of microbial infections in the body are attributed to biofilms [13]. Biofilms are accumulations of microorganisms embedded in a polysaccharide matrix that are adherent to solid surfaces, often resulting in an infection which further complicates recovery and impacts quality of life [13-16]. Furthermore, innate to biofilm formation is antibiotic tolerance and even resistance, ultimately resulting in recalcitrant infection [16-18]. Recalcitrant infection often requires revision surgeries, which when possible will include removal and replacement of the contaminated hardware. However, unlike in joint where implants can be replaced by non-functional antibiotic elution systems, the spinal site requires the stabilization afforded by the hardware, making treatment especially complicated [19, 20]. Since prevention is the best strategy, we have sought to augment the antibiotic prophylaxis already in place to further decrease infection rates. Therefore, we have designed and evaluated an ultrasound-activated bulk release system to combat surgical site infections.
The continued presence of the contaminating bacteria is often associated with non-genetic changes resulting in the acquisition of a persister phenotype that can survive the initial high levels of antibiotics and then start to proliferate once the prophylactic doses have dropped below therapeutic levels [21]. We hypothesize that those surviving pathogens can be eradicated by a second, delayed, post-surgical delivery of supra-therapeutic concentrations of mixed prophylactic antibiotics within the spinal fusion site, with respect to the minimum inhibitory concentration (MIC) of the invading microbes, thus lowering postoperative infection rates. The MIC for VAN against Staphylococcus aureus, one of the most common microbes associated with implant-associated infection [22, 23], is 2 μg/mL [24, 25].
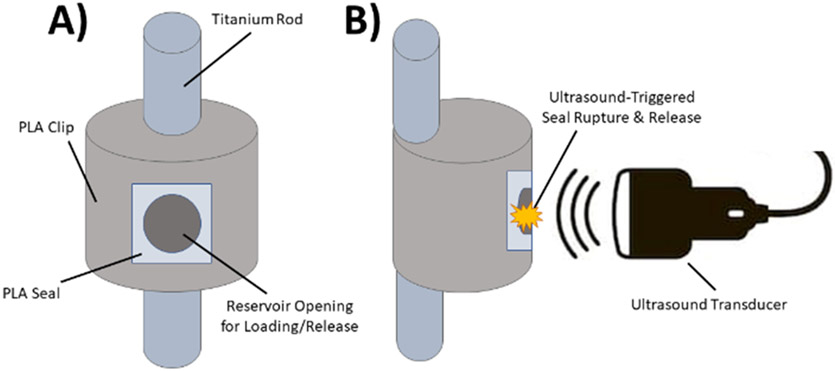
We developed a polylactic acid (PLA) reservoir clip that affixes to a standard spinal rod and serves as a non-eluting reservoir of prophylactic antibiotics [14, 17]. At the surgeon’s direction, at least 3 days following surgery when prophylactic antibiotics are depleted and surgical drains are removed, this reservoir can be activated by application of transcutaneous ultrasound, causing rupture of the PLA film sealing the reservoir. Microbubbles, added to the antibiotic solution as additional cavitation nuclei, can aid in the process of rupturing the PLA membrane film. This rupture triggers a local, bolus release of supra-therapeutic levels of antibiotics, far above the MIC, to the surgical site. A representative diagram of this delivery system is shown in Figure 1.
Figure 1:
Diagram of clip system (not to scale). A) Frontal view of clip system. B) Side view of clip system during ultrasound-induced PLA film rupture.
The purpose of this study was to perform in vitro and in vivo evaluations of this ultrasound-triggered delivery system. First, the long-term stability of Sonazoid microbubbles within the VAN solution was quantified to confirm their viability for inclusion in the device. Then, we quantified the ultrasound-triggered VAN release from this system in vitro. Finally, the safety and activation functionality of this device was evaluated in vivo using two animal models.
Materials & Methods
Polymer Clip Preparation
The PLA clips were designed with a C-shape that was specifically designed to stably clip onto standard spinal fusion hardware. The clip contained an inner drug reservoir, as described previously [14, 17]. Briefly, the 1.0 cm3 clips were 3D printed using a 2.85 mm, 750 g PLA filament (Ultimaker, Utrecht, The Netherlands) to surround a 0.3 cm3 drug reservoir able to contain therapeutically relevant amounts of prophylactic drug. The outer-facing curved surface of the clip was designed with a 4-mm circular opening for loading the deliverable and its subsequent ultrasound-triggered release.
For the in vitro evaluations, VAN was used in the clips as it is one of the drugs used in clinical practice. A VAN suspension (Athenex, Buffalo, NY) was prepared with 1 g VAN in 2.5 mL distilled water for a final concentration of 400 mg VAN/mL. Water was used as the suspension medium to simulate clinical re-suspension of powdered VAN. Clips were loaded with 200 μL of the VAN suspension (80 mg total VAN loading) and 50 μL of Sonazoid microbubbles (GE HealthCare, Oslo, Norway) which acted as cavitation nuclei aiding in the rupture of the PLA film seal and release of the VAN. Once loaded, the clips were sealed with a hand-cast PLA (Resomer Select 100 DL 7E; Evonik Industries, Essen, Germany) film (0.05 ± 0.01 mm thick) secured to the clip by brushing with chloroform (Fisher Scientific, Hampton, NH). All clips were dried under continuous air-flow at room temperature for 24 hours. Coated, loaded clips were kept at 4°C until used for testing.
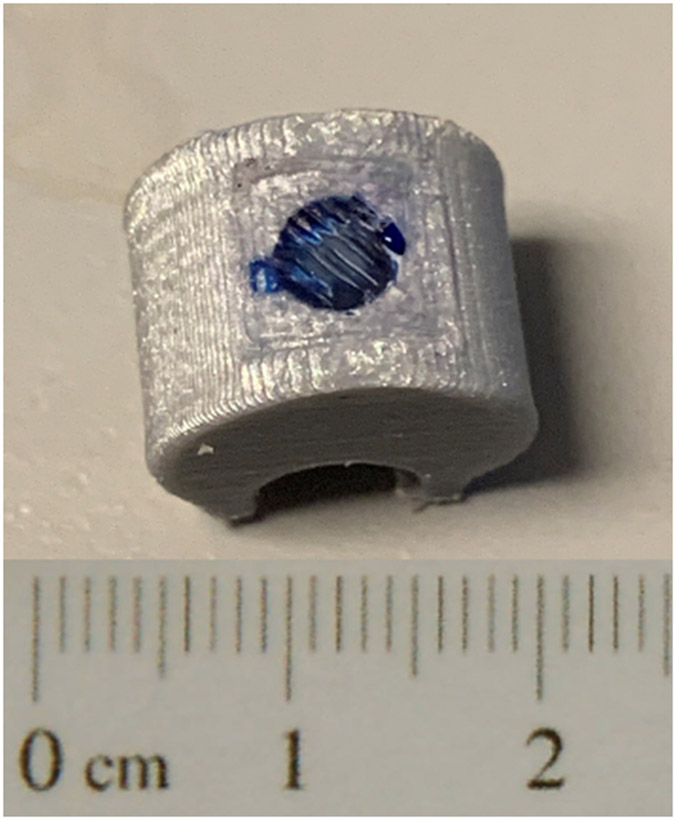
For the in vivo evaluations, methylene blue (MeB; Sigma Aldrich, St. Louis, MO), an intense dye that is commonly used to simulate drug release [14, 26, 27], was used in the clips to easily visualize ultrasound-triggered release as detected by blue staining of the surrounding tissues. Clips were loaded with 200 μL of a MeB slurry and 50 μL of Sonazoid microbubbles, then sealed and stored as described previously. A representative image of a MeB-loaded, PLA-sealed clip is shown in Figure 2.
Figure 2:
Example of MeB-loaded, PLA-sealed clip with scale bar.
Long-term Sonazoid Stability
Long-term stability of Sonazoid microbubbles was determined over a 14-day incubation at three temperatures that represent different stages of use: 4°C to mimic storage conditions of prepared clips prior to implantation, 25°C to mimic conditions during surgical procedures, and 37°C to mimic implantation conditions. Briefly, Sonazoid was reconstituted in sterile water per manufacturer’s instructions. Reconstituted Sonazoid (50 μL) was added to 150 μL of either distilled water or VAN suspension (Athenex, 400 mg VAN/mL distilled water) in Eppendorf tubes and stored at 4°C, 25°C, and 37°C. Microbubble counts and diameter were obtained in triplicate after 0, 1, 3, 6, 8, 10, and 14 days using a PSS AccuSizer FX Nano (Entegris, Billerica, MA) using an LE sensor with a range of 0.5 to 200 μm. Contrast enhancement of the Sonazoid solution was evaluated on days 0, 3, 8, and 14 using a Logiq E10 ultrasound scanner (GE HealthCare) in contrast mode (transmitting at 5.0 MHz at an MI of 0.12), and quantified using ImageJ software (NIH, Bethesda, MD). Statistical analysis, including ANOVA and paired and unpaired Student’s t tests between groups, was performed with Prism 8 (GraphPad, La Jolla, CA) and assuming α < 0.05 indicates statistical significance.
In Vitro Quantification of VAN Release
Loaded, sealed clips (n = 5) were submerged in water heated to 37°C to mimic the biological environment, and insonated for 10 minutes using a Logiq E9 clinical ultrasound scanner (GE HealthCare) equipped with a C1-6 curvilinear probe using previously optimized ultrasound parameters [14]. Grayscale imaging (4.0 MHz) was used to establish the positioning of the clip within the focal zone, and then power Doppler imaging (1.8 MHz frequency, 6.5 kHz pulse repetition frequency, mechanical index (MI) 1.2, intensity (ISPTA) 146.2 ± 1.4 mW/cm2) was used to induce rupture of the PLA seal. Resulting VAN release was quantified using spectrophotometry (Tecan, Männedorf, Switzerland; 281 nm absorbance) at t = 0, 10, 20, 30, 40, 50, 60 minutes, then at 2, 4, 6, 12, 24, 36, 48, and 72 hours. Control clips (n = 5) were submerged in the water bath but not insonated to evaluate for any passive leakage. Readings were taken in triplicate, and results are reported as mean ± standard deviation. Statistical analysis using unpaired Student’s t tests between groups was performed with Prism 8 and assuming α < 0.05 indicates statistical significance.
In Vivo Evaluation of Ultrasound-Triggered Rupture
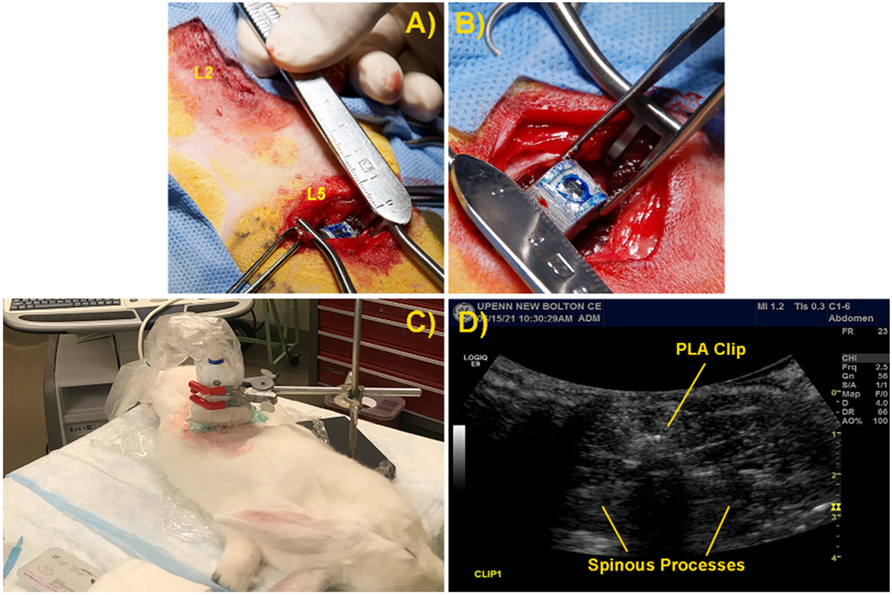
Clips were sterilized with cold ethylene oxide prior to loading and coating under aseptic conditions. MeB-loaded, PLA-sealed clips were implanted into three skeletally mature New Zealand White rabbits (4-5 kg) along the spinal midline at the L2 and L5 levels, as well as one male, castrated, juvenile Yorkshire pig (40 kg) at the L1 and L3 levels, under IACUC approved protocols. Briefly, under endotracheal anesthesia using isoflurane gas inhalation mixed with oxygen, the surgical site was prepared for orthopedic surgery. The dorsal spinous processes were exposed via a curvilinear incision, the dorsal spinous process was resected, and the clip was implanted in the resulting dead space (Figure 3A and B). Following recovery from anesthesia, animals were returned to their respective vivarium with unrestricted access to exercise.
Figure 3:
Implantation and insonation of clip within rabbit model. A) Location of surgical site abaxial to midline of rabbit spine. B) Implantation of loaded and sealed clip into rabbit spinal wound site. C) Insonation of rabbit to induce clip rupture and MeB release. D) Ultrasound image of clip within rabbit spinal surgical site.
Two of the rabbits were used as controls to determine the minimum longevity and confirm the retention of the encapsulated payload within the clips in the absence of ultrasound. One rabbit was euthanized 3 days post-operative and the other was euthanized 5 days post-operative, and implants with surrounding soft tissues were harvested for analysis.
Four days postoperative, the third rabbit and the pig were re-anesthetized and insonated in Doppler mode at 1.7 MHz for 20 minutes using a Logiq E9 scanner (GE HealthCare) with a C6 probe (5.4 kHz PRF, MI 1.2, ISPTA < 146 mW/cm2) to rupture the PLA seal and induce bolus release (Figure 3C and D) [14]. Both clips were insonated in the rabbit, while only one clip was insonated in the pig with the other remaining clip uninsonated as a control. Animals were sacrificed 2 hours post-insonation and implants with surrounding soft tissues were harvested for analysis.
After sacrifice and clip retrieval, surgical sites were photographed and assessed for MeB staining. The retrieved clips were first visually inspected for PLA film rupture. Then, the retrieved clips were submerged in a water bath and observed for any release of MeB from the reservoir. Following this submersion, the clips were removed from the water bath, the PLA film was punctured with a needle to ensure catastrophic rupture of the film, and then the clips were re-submerged in a fresh water bath to visualize any release of material from the internal reservoir. This process was followed to evaluate payload retention in the absence of visible film rupture. Before and after photos were taken of each clip and water bath submersion.
Results
Long-term Sonazoid Stability
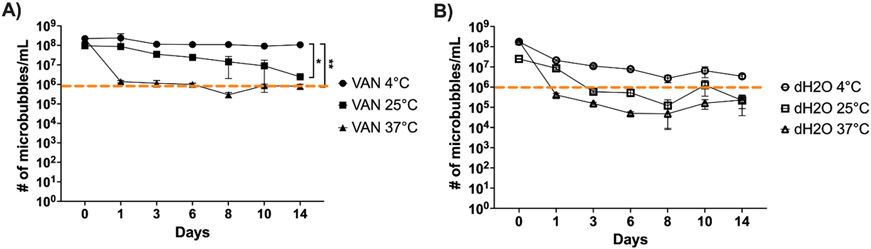
At all three temperatures evaluated, microbubble survival over the 14-day incubation differed between distilled water and VAN suspension (Figure 4). Based on visual evaluation of the samples, together with the contrast imaging evaluation, a microbubble count of 106 or higher indicates the presence of viable microbubbles, while a count of below 106 indicates debris and non-viable microbubbles.
Figure 4:
Graph of Sonazoid longevity in VAN solution and distilled water (dH2O) at 4°C, 25°C, and 37°C in Log10 scale over 14 days; A) Sonazoid in VAN solution, *p = 0.0159, **p = 0.0104; B) Sonazoid in distilled water; dotted orange line indicates cutoff between viable microbubbles and non-viable debris as observed visually at 106; error bars represent standard deviation; n = 3.
In VAN solution (Figure 4A), Sonazoid was relatively well-preserved over 14 days at both 4°C (from 2.26 x 108 ± 3.97 x 107 bubbles/mL to 1.08 x 108 ± 1.03 x 107 bubbles/mL, p = 0.0451) and 25°C (from 9.64 x 107 ± 1.65 x 107 bubbles/mL to 2.46 x 106 ± 2.96 x 105 bubbles/mL, p = 0.0047). At 37°C, however, there was a more dramatic loss of microbubbles (from 2.17 x 108 ± 9.07 x 106 bubbles/mL to 7.96 x 105 ± 2.10 x 105 bubbles/mL, p < 0.0001), resulting in mostly debris being present by Day 6. Incubation at 4°C proved to be more conservatory than both 25°C (p = 0.0159) and 37°C (p = 0.0104). There was no significant difference in microbubble degradation between 25°C and 37°C (p = 0.98).
There was a larger reduction in the number of viable Sonazoid microbubbles in distilled water, where the number of microbubbles was reduced by 2 to 3 orders of magnitude across all 3 temperatures (Figure 4B). Some viable microbubbles were retained over 14 days at 4°C (from 1.76 x 108 ± 4.51 x 106 bubbles/mL to 3.46 x 106 ± 7.89 x 105 bubbles/mL, p < 0.0001). However, samples were reduced to mostly debris in the 25°C group by Day 3 (from 2.51 x 107 ± 7.55 x 105 bubbles/mL to 2.22 x 105 ± 1.83 x 105 bubbles/mL over 14 days, p < 0.0001), and in the 37°C group by Day 1 (from 1.86 x 108 ± 2.68 x 107 bubbles/mL to 2.32 x 105 ± 1.45 x 105 bubbles/mL, p = 0.0023). None of the incubation temperatures resulted in significantly different degradation curves (p = 0.62).
At all temperatures, VAN appears to have a conservatory effect on reconstituted Sonazoid microbubbles (p = 0.0131). Importantly, there were no significant changes to the average microbubble diameter (1.40 ± 0.13 μm across all groups) over the 14 days for any of the six incubation conditions (p > 0.80).
Representative images of Sonazoid microbubbles in VAN solution at 4°C across the 14-day incubation are shown in Figure 5. Sonazoid incubated in the VAN solution exhibited a 5.44% decrease in enhancement intensity at Day 14 (from 116.09 ± 0.81 AU at Day 0 to 109.77 ± 0.25 AU at Day 14, p < 0.0001). Similarly, incubation in distilled water resulted in a 4.70% reduction in enhancement intensity (from 116.25 ± 0.45 to 110.79 ± 0.65 AU, p < 0.0001) over the 14 days. However, unlike the number of microbubbles, there was no difference in the intensity levels between distilled water and VAN at Day 0 (p > 0.99) or Day 14 (p = 0.63). Importantly, marked contrast enhancement was observed from both the distilled water and VAN solution at the 4 imaging time points, indicating the remaining microbubbles were still acoustically active.
Figure 5:
Composite of contrast images showing Sonazoid longevity in VAN solution at 4°C over 14 days. Left: Baseline images prior to injection of Sonazoid solution. Right: Images following injection of Sonazoid solution in sample tank.
In Vitro Quantification of VAN Release
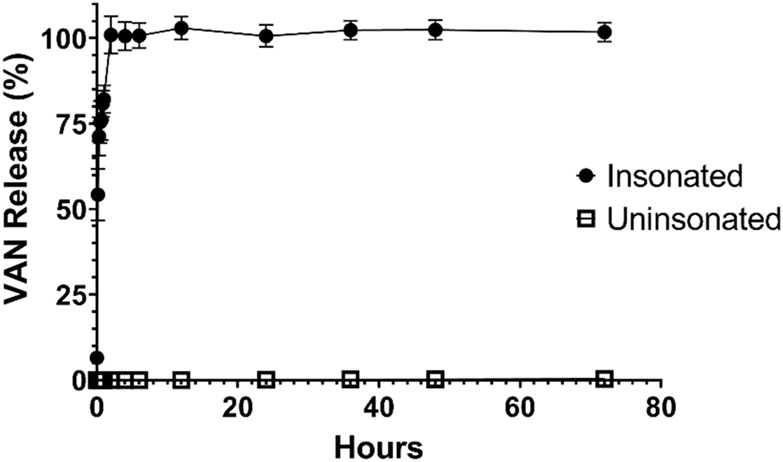
Ultrasound-triggered rupture of the PLA seal was observed in all 5 insonated clips, while the 5 uninsonated clips showed no visible evidence of PLA film rupture or compromise. The insonated clips exhibited an average cumulative VAN release of 81.4 ± 2.8 mg (101.8 ± 2.8% of initial loading) by 72 hours (Figure 6), whereas the uninsonated clips exhibited 0.3 ± 0.1 mg (0.3 ± 0.1% of initial loading) by 72 hours (p < 0.0001 compared to insonated group). Approximately 66 mg (82.1 ± 4.0%) of the loaded VAN solution was released from the insonated clips within 1 hour following exposure to ultrasound, with 100.9 ± 5.5% of the VAN released by 2 hours (80.8 mg, p = 0.82 compared to 72 hours), supporting the bolus release of the supratherapeutic dose to the wound site.
Figure 6:
Graph of average cumulative VAN in vitro release in mg from insonated and uninsonated clips; error bars represent standard deviation; n = 5 in each group.
In Vivo Evaluation of Ultrasound-Triggered Rupture
All animals recovered uneventfully from the index procedure. The rabbit died during anesthetic induction for the insonation procedure, but the implanted clips were still insonated and included in the study. The pig tolerated the insonation procedures with no adverse effects.
The uninsonated clips that were retrieved from the two control rabbits showed no visible signs of PLA film compromise or rupture, and there was no visible MeB staining in the tissues surrounding the clips (results not shown for the sake of brevity). These findings suggest that the clips maintain their integrity in vivo for at least 5 days post-operatively in the absence of insonation.
The two insonated clips that were retrieved from the third rabbit spine showed no visible signs of PLA film rupture. Additionally, there was no visible MeB staining in the tissues surrounding the clip (Figure 7A). When the retrieved clips were submerged in the water bath, there was no visible release of MeB solution from within the clip (Figure 7B). Following the physical puncture of the PLA film with a needle (Figures 7C and D), release of MeB solution from within the reservoir was clearly visible upon re-submersion in the water bath (Figure 7E). This suggests that the ultrasound-triggered rupture of the PLA film was unsuccessful in the rabbit model.
Figure 7:
Composite of in vivo evaluation of explants from rabbit. A) Explantation at end-term surgery 2 hours post-insonation. B) Submersion of retrieved clip in water bath showing no MeB release from clip. C and D) Physical puncture of PLA film covering clip reservoir. E) Submersion of physically punctured clip in water bath showing MeB release from clip.
The pig model, however, exhibited ultrasound-triggered rupture of the insonated clip. In situ inspection of the surgical sites and the explanted clips from the pig showed visible MeB staining in the overlaying and surrounding tissues (Figures 8A and B), as well as a visible hole in the PLA film (Figure 8C), at the site that was insonated. Additionally, this clip exhibited no release of MeB solution during the initial water bath submersion nor when re-submerged (Figure 8D, left tube), demonstrating that the MeB solution was completely released in vivo in response to the insonation. Conversely, the uninsonated clip showed no signs of PLA film rupture nor any staining of the surrounding tissues (not pictured). After physical puncture of the PLA film with a needle, there was visible release of MeB solution from the uninsonated clip upon re-submersion in the water bath (Figure 8D, right tube). Therefore, the clip successfully retained the payload in the absence of ultrasound triggering.
Figure 8:
Composite of in vivo evaluation of explants from the pig model. A) Removal of MeB stained overlying tissues from insonated wound site. B) Visible MeB staining of tissue surrounding insonated clip, arrow shows compromised PLA film. C) Visible PLA film rupture from clip that was insonated. D) Water bath post-physical puncture of PLA film showing no further MeB release from insonated clip (left) but positive MeB release from uninsonated clip (right).
Discussion
Overall, we have demonstrated the ability to produce ultrasound-triggered release of an encapsulated prophylactic drug from within 3D-printed PLA clips. The Sonazoid microbubbles maintained their functionality over 14 days in simulated storage at 4°C, representing the maximum expected shelf life of these devices, as well as viability at 37°C for up to 6 days, supporting their inclusion within the device to assist with the ultrasound-triggered rupture of the PLA seal. We also demonstrated that the PLA seal was susceptible to ultrasound-triggered rupture both in vitro and via percutaneous insonation in vivo in the pig model. Concurrently, uninsonated clips maintained their integrity and payload both in vitro and in vivo. These results demonstrate an important proof of concept for continuing in vivo evaluations in large animal models to demonstrate clinical utility.
Clinical treatment of spinal infection continues to be fraught by setbacks, relying on intravenous systemic antibiotics as a first-line treatment method, with an additional local prophylaxis often initiated [7, 28-31]. However, once a surgical site infection has established, systemic intravenous antimicrobials often fail at eradicating the infection. Moreover, implant-associated infections frequently involve recalcitrant biofilms colonizing the implant surfaces, typically requiring aggressive revision surgeries with debridement and hardware modification or replacement [30, 31]. There are relatively few interventions that rely on hardware modification during revision surgery, with the most common method being application of antibiotic-loaded bone cement. As an example, Masuda et al. have developed an antibiotic-loaded polymethylmethacrylate (PMMA) bone cement that is used to coat the instrumentation during revision surgery and provide localized antibiotic release in patients with intractable, complex infection following instrumented spinal surgery [32]. The PMMA was loaded at 10% by weight with a combination of VAN and amikacin, with the ratio of each antibiotic being tailored to the bacteria responsible for the infection [32]. Ten of 11 patients resolved infection after 2-6 weeks and 1 patient required a second coating of drug-laden cement for resolution of infection [32]. While this method was successful for treating these established infections, it is invasive and results in increased cost to the healthcare system and prolonged pain and suffering for the patients. The drug delivery device presented in this paper aims to prevent infection of spinal hardware from developing and becoming intractable, while also preventing the need for reopening the wound and increasing the chances of introducing additional invading microbes.
The impetus for developing this ultrasound-triggered antibiotic delivery system was based on the observation that peri-surgical infection occurred in up to 20% of cases, despite aggressive peri-operative prophylaxis [5, 7, 33]. It is thought that the antibiotics placed in the wound site peri-operatively are depleted by the presence of aseptic drains for the first 2-3 days following surgery [17]. We therefore reasoned that a second spatiotemporal bolus dose of prophylactic antibiotics at the wound site could eradicate bacteria that eluded the initial perioperative treatment, whether through physical escape or “persister” phenotype [17, 34, 35]. Our device is designed such that it can house and deliver either a single or combination of antibiotics for broad-spectrum coverage of invading microbes. By delivering the prophylactic payload at least 3 days after surgery, any remaining bacteria have sufficient time to transition back from the persister phenotype to one more sensitive to antibiotic treatment [17, 34, 35]. Additionally, burst release is preferable compared to slow elution, as slow elution could introduce selective pressure, allowing for development of antibacterial resistance [34, 35]. Importantly, we observed that the VAN payload was retained within the clips prior to ultrasound triggering, which would allow time for any remaining bacteria in the surgical site to return to the susceptible phenotype. We did measure a small amount of VAN from uninsonated clips, and suggest that this small amount resulted from residual dried VAN on the exterior of the clip that was deposited during the loading and coating process, and not any active leakage as the films were visually intact at the conclusion of the experiment. Additionally, we observed that approximately 80% of the encapsulated VAN was released within the first hour post-insonation and 99% was released within the first two hours post-insonation. As mentioned previously, S. aureus is one of the main invading microbes responsible for implant-associated infection [22, 23], which guided our choice of VAN as the payload in these clips. Guidelines provided by the Clinical Laboratory Standards Institute (CLSI) and European Committee on Antimicrobial Testing (EUCAST) define VAN-sensitive S. aureus as having an MIC ≤ 2 μg/mL, while an MIC between 4-8 μg/mL is considered vancomycin-intermediate S. aureus (VISA) and greater than 16 μg/mL is widely considered VAN resistance (VRSA) [24, 25, 36, 37]. Importantly, there is recent discussion as to whether the classical MIC is an effective measure, or whether the minimum concentrations for biofilm inhibition (MBIC) and biofilm eradication (MBEC) should also be considered [38]. We have demonstrated that these clips release ≥ 100 ug/mL VAN to the area surrounding the device, both in this and previous studies [17]. Therefore, the bolus release profile from these clips is amenable to bacterial eradication as a second-wave treatment.
An unexpected finding during the longevity study was that VAN had a conservatory effect on the Sonazoid microbubbles compared to sterile water, especially at 37°C (cf., Figure 4). We hypothesize that this extended viability and longevity was due to the higher viscosity of the VAN suspension. It is well documented that higher viscosity in the surrounding medium has several effects on microbubbles, including differences in three-dimensional motion, deformation, and dampening of microbubble oscillations [39-42]. Importantly, Abou-Selah et al. demonstrated that higher viscosity medium led to reduced gas exchange and increased microbubble longevity [43]. While there was a large drop-off in the number of Sonazoid microbubbles in VAN at 37°C over the first 6 days of incubation, we do believe that some microbubbles remained viable within our clips and helped to facilitate the ultrasound-triggered rupture of the PLA film. However, the inclusion of these cavitation nuclei may be less important than we previously thought, and warrants further investigation in future in vivo studies.
We used MeB as a model drug for the in vivo studies to better visualize release and disposition within the surrounding tissues. MeB is an intense dye that is commonly used to visualize drug release [14, 26, 27], and provided a simple and unambiguous method to evaluate these devices for successful rupture. Previously, we were able to successfully induce ultrasound-triggered PLA film rupture in an ex vivo model using optimized ultrasound parameters [14, 17]. However, in the present in vivo rabbit model, we were unable to successfully rupture the PLA film and induce release of the encapsulated MeB solution via a transcutaneous approach. We believe that this may be a size issue. The ratio of available space following dorsal spinous resection and the size of the clip prevent any dead space around the clip. Dead space (approximately 1-2 cm3 in size) commonly develops during surgical procedures and generally fills with wound fluid and exudate during the acute healing phase. Thus, diligent management of dead space is a guiding surgical principle. The spinal topography often results in residual dead space following posterior surgical interventions in larger animals and humans [44]. Lack thereof in the rabbit model resulted in an intimate contact of the overlaying soft tissue layers, which appeared to prevent cavitation and rupture of the PLA film, as one of the most important aspects of the ultrasound-triggered delivery is the vibration and cavitation of the PLA film itself. We thus turned to the larger pig model to better represent the proportion of dead space in relation to the implant, mimicking the surgical site during human spinal fusion surgery [44]. As such, we were able to successfully induce ultrasound-triggered rupture of the PLA film as well as subsequent release of the encapsulated MeB in the pig model, while the uninsonated clip remained intact. These findings suggest that this larger animal model will be more appropriate for continuing in vivo evaluation of this delivery system, including the addition of spinal instrumentation from the human armamentarium.
The study findings presented in this paper are encouraging, but there are several limitations. One limitation to the study was a lack of fluid flow, as well as biologically-relevant enzymes and proteins, in the surrounding media during the in vitro elution experiments. However, we believe that the observed integrity of the uninsonated clip in the pig model, which was susceptible to fluid flow, enzymes, and proteins, provides appropriate evidence that the PLA film is not structurally or functionally compromised by these elements that were missing from the in vitro evaluations. We also acknowledge that the contrast imaging performed on Sonazoid incubated at 4°C could be different from those at 25°C and 37°C. However, we chose to focus on the 4°C incubation, as we expect this to be the longest incubation condition when clips are stored in the interval between loading/preparation and implantation. Another important limitation to this study was the small sample size for the in vivo evaluations. Following the 3 Rs of responsible in vivo research, we selected the rabbit model as the lowest vertebrate model possible for this implant. Once we determined that this model did not afford an appropriate evaluation of the clip, we resorted to a larger animal model (i.e., the pig). Pigs are frequently used in spinal surgery, and thus presented with a realistic surgical site including substantial amounts of dead space akin to a surgical site in a human patient. Given our extensive in vitro data, combined with our robust proof-of-concept findings from the pig model experiment, we are confident that we have established sufficient translational merit in support of continued translation towards clinical utility. Finally, we acknowledge that next steps have to include experiments investigating local tissue concentration of the delivered payload as well as demonstrating efficacy using a bacterial infection challenge model in the presence of spinal hardware, both in vitro and in vivo. This will be necessary to fully characterize the active antibiotic release profile and to demonstrate effective bacterial eradication using this technology, acknowledging that in our in vivo studies complete independence between surgical sites is assumed but cannot be fully guaranteed. Despite these limitations, the results of this study justify continued exploration of this physician-triggered prophylactic delivery system.
Conclusion
Current methods for preventing peri-operative spinal implant infection are only partially successful, with incidences of up to 20%. To prevent this clinical problem, we have designed an ultrasound-triggered drug delivery system that can carry a range of prophylactic antibiotics i.e., active against both Gram-positive and Gram-negative pathogens, to aggressively combat post-surgical bacterial survival without additional invasive post-surgical intervention. Here, we have demonstrated the in vitro and in vivo functionality of this ultrasound-triggered delivery system. The ultimate translational goal of this delivery system is to provide spine surgeons with an effective tool to further minimize the risk of post-operative spinal implant infection.
Acknowledgements
The authors would like to thank Dr, Margaret Wheatley, Dr. Daniel MacDonald, and Kathleen Gillmore for their contributions. Additionally, we thank GE HealthCare for equipment support. The work presented in this manuscript is funded by NIH grant R01 AR069119. Lauren Delaney was funded by NIH fellowship F32 AR072491 and K99 AR078354.
Footnotes
Conflict of Interest: All relevant conflict of interest information has been provided in the ICMJE Conflict of Interest forms for each author.
References
- [1].Omidi-Kashani F, Hasankhani EG, Ashjazadeh A. Lumbar spinal stenosis: who should be fused? An updated review. Asian Spine J. 2014;8:521–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Deng H, Yue JK, Ordaz A, Suen CG, Sing DC. Elective lumbar fusion in the United States: national trends in inpatient complications and cost from 2002-2014. J Neurosurg Sci. 2021;65:503–12. [DOI] [PubMed] [Google Scholar]
- [3].Beschloss A, Ishmael T, Dicindio C, Hendow C, Ha A, Louie P, et al. The Expanding Frontier of Outpatient Spine Surgery. Int J Spine Surg. 2021;15:266–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Moyano CA, Tello CA, Piantoni L, Wilson IAF, Galaretto E, Remondino RG, et al. Infection Recurrence in Instrumented Spinal Fusion in Children. Global Spine J. 2021;11:1040–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Schömig F, Gogia J, Caridi J. Epidemiology of postoperative spinal implant infections. J Spine Surg. 2020;6:762–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kim BD, Hsu WK, De Oliveira GS Jr., Saha S, Kim JYS. Operative Duration as an Independent Risk Factor for Postoperative Complications in Single-Level Lumbar Fusion: An Analysis of 4588 Surgical Cases. Spine. 2014;39. [DOI] [PubMed] [Google Scholar]
- [7].O'Neill KR, Smith JG, Abtahi AM, Archer KR, Spengler DM, McGirt MJ, et al. Reduced surgical site infections in patients undergoing posterior spinal stabilization of traumatic injuries using vancomycin powder. Spine J. 2011;11:641–6. [DOI] [PubMed] [Google Scholar]
- [8].Francis AM, Mericli AF. Spine Reconstruction: From Basics to Cutting Edge. Current Surgery Reports. 2022;10:255–64. [Google Scholar]
- [9].Bhavan KP, Marschall J, Olsen MA, Fraser VJ, Wright NM, Warren DK. The epidemiology of hematogenous vertebral osteomyelitis: a cohort study in a tertiary care hospital. BMC Infect Dis. 2010;10:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Loibl M, Stoyanov L, Doenitz C, Brawanski A, Wiggermann P, Krutsch W, et al. Outcome-related co-factors in 105 cases of vertebral osteomyelitis in a tertiary care hospital. Infection. 2014;42:503–10. [DOI] [PubMed] [Google Scholar]
- [11].Legrand E, Flipo RM, Guggenbuhl P, Masson C, Maillefert JF, Soubrier M, et al. Management of nontuberculous infectious discitis. treatments used in 110 patients admitted to 12 teaching hospitals in France. Joint Bone Spine. 2001;68:504–9. [DOI] [PubMed] [Google Scholar]
- [12].Cebrián Parra JL, Saez-Arenillas Martín A, Urda Martínez-Aedo AL, Soler Ivañez I, Agreda E, Lopez-Duran Stern L. Management of infectious discitis. Outcome in one hundred and eight patients in a University Hospital. International Orthopaedics. 2012;36:239–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].National Institutes of Health. Research on Microbial Biofilms. Bethesda, MD;2002. [Google Scholar]
- [14].Delaney LJ, Basgul C, MacDonald DW, Fitzgerald K, Hickok NJ, Kurtz SM, et al. Acoustic Parameters for Optimal Ultrasound-Triggered Release from Novel Spinal Hardware Devices. Ultrasound Med Biol. 2020;46:350–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nature reviews microbiology. 2004;2:95–108. [DOI] [PubMed] [Google Scholar]
- [16].LuTheryn G, Glynne-Jones P, Webb JS, Carugo D. Ultrasound-mediated therapies for the treatment of biofilms in chronic wounds: a review of present knowledge. Microbial Biotechnology. 2020;13:613–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Delaney LJ, MacDonald D, Leung J, Fitzgerald K, Sevit AM, Eisenbrey JR, et al. Ultrasound-triggered antibiotic release from PEEK clips to prevent spinal fusion infection: Initial evaluations. Acta Biomater. 2019;93:12–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wolcott RD, Rhoads DD, Dowd SE. Biofilms and chronic wound inflammation. Journal of wound care. 2008;17:333–41. [DOI] [PubMed] [Google Scholar]
- [19].Collins I, Wilson-MacDonald J, Chami G, Burgoyne W, Vineyakam P, Berendt T, et al. The diagnosis and management of infection following instrumented spinal fusion. European Spine Journal. 2008;17:445–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mok JM, Guillaume TJ, Talu U, Berven SH, Deviren V, Kroeber M, et al. Clinical outcome of deep wound infection after instrumented posterior spinal fusion: a matched cohort analysis. Spine. 2009;34:578–83. [DOI] [PubMed] [Google Scholar]
- [21].Saeed K, McLaren AC, Schwarz EM, Antoci V, Arnold WV, Chen AF, et al. 2018 international consensus meeting on musculoskeletal infection: Summary from the biofilm workgroup and consensus on biofilm related musculoskeletal infections. Journal of Orthopaedic Research. 2019;37:1007–17. [DOI] [PubMed] [Google Scholar]
- [22].Oliveira WF, Silva PMS, Silva RCS, Silva GMM, Machado G, Coelho L, et al. Staphylococcus aureus and Staphylococcus epidermidis infections on implants. J Hosp Infect. 2018;98:111–7. [DOI] [PubMed] [Google Scholar]
- [23].Foster CE, Lamberth LB, Kaplan SL, Hulten KG. Clinical Characteristics and Outcomes of Staphylococcus aureus Implant-associated Infections in Children. Pediatr Infect Dis J. 2019;38:808–11. [DOI] [PubMed] [Google Scholar]
- [24].Moses VK, Kandi V, Rao SKD. Minimum Inhibitory Concentrations of Vancomycin and Daptomycin Against Methicillin-resistant Staphylococcus Aureus Isolated from Various Clinical Specimens: A Study from South India. Cureus. 2020;12:e6749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Shariati A, Dadashi M, Moghadam MT, van Belkum A, Yaslianifard S, Darban-Sarokhalil D. Global prevalence and distribution of vancomycin resistant, vancomycin intermediate and heterogeneously vancomycin intermediate Staphylococcus aureus clinical isolates: a systematic review and meta-analysis. Scientific reports. 2020;10:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bikram M, Gobin AM, Whitmire RE, West JL. Temperature-sensitive hydrogels with SiO2–Au nanoshells for controlled drug delivery. Journal of Controlled Release. 2007;123:219–27. [DOI] [PubMed] [Google Scholar]
- [27].Satarkar NS, Hilt JZ. Magnetic hydrogel nanocomposites for remote controlled pulsatile drug release. Journal of Controlled Release. 2008;130:246–51. [DOI] [PubMed] [Google Scholar]
- [28].Tsantes AG, Papadopoulos DV, Vrioni G, Sioutis S, Sapkas G, Benzakour A, et al. Spinal infections: an update. Microorganisms. 2020;8:476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Daldal I, Senkoylu A. Strategies of management of deep spinal infection: from irrigation and debridement to vacuum-assisted closure treatment. Annals of Translational Medicine. 2020;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lener S, Hartmann S, Barbagallo GMV, Certo F, Thomé C, Tschugg A. Management of spinal infection: a review of the literature. Acta Neurochirurgica. 2018;160:487–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Dowdell J, Brochin R, Kim J, Overley S, Oren J, Freedman B, et al. Postoperative spine infection: diagnosis and management. Global spine journal. 2018;8:37S–43S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Masuda S, Fujibayashi S, Otsuki B, Kimura H, Matsuda S. Efficacy of target drug delivery and dead space reduction using antibiotic-loaded bone cement for the treatment of complex spinal infection. Clinical spine surgery. 2017;30:E1246–E50. [DOI] [PubMed] [Google Scholar]
- [33].Ghobrial GM, Thakkar V, Andrews E, Lang M, Chitale A, Oppenlander ME, et al. Intraoperative vancomycin use in spinal surgery: single institution experience and microbial trends. Spine. 2014;39:550–5. [DOI] [PubMed] [Google Scholar]
- [34].Lewis K. Multidrug tolerance of biofilms and persister cells. Bacterial biofilms. 2008:107–31. [DOI] [PubMed] [Google Scholar]
- [35].Dastgheyb SS, Hammoud S, Ketonis C, Liu AY, Fitzgerald K, Parvizi J, et al. Staphylococcal persistence due to biofilm formation in synovial fluid containing prophylactic cefazolin. Antimicrobial agents and chemotherapy. 2015;59:2122–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kest H, Kaushik A. Vancomycin-resistant Staphylococcus aureus: Formidable threat or silence before the storm. J Infect Dis Epidemiol. 2019;5:93. [Google Scholar]
- [37].Mancuso G, Midiri A, Gerace E, Biondo C. Bacterial Antibiotic Resistance: The Most Critical Pathogens. Pathogens. 2021;10:1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Thieme L, Hartung A, Tramm K, Klinger-Strobel M, Jandt KD, Makarewicz O, et al. MBEC Versus MBIC: the Lack of Differentiation between Biofilm Reducing and Inhibitory Effects as a Current Problem in Biofilm Methodology. Biological Procedures Online. 2019;21:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].De Jong N, Bouakaz A, Frinking P. Basic Acoustic Properties of Microbubbles. Echocardiography. 2002;19:229–40. [DOI] [PubMed] [Google Scholar]
- [40].Helfield B, Black JJ, Qin B, Pacella J, Chen X, Villanueva FS. Fluid Viscosity Affects the Fragmentation and Inertial Cavitation Threshold of Lipid-Encapsulated Microbubbles. Ultrasound in Medicine & Biology. 2016;42:782–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Manmi K, Wang Q. Acoustic microbubble dynamics with viscous effects. Ultrasonics Sonochemistry. 2017;36:427–36. [DOI] [PubMed] [Google Scholar]
- [42].Klaseboer E, Manica R, Chan DYC, Khoo BC. BEM simulations of potential flow with viscous effects as applied to a rising bubble. Engineering Analysis with Boundary Elements. 2011;35:489–94. [Google Scholar]
- [43].Abou-Saleh RH, McLaughlan JR, Bushby RJ, Johnson BR, Freear S, Evans SD, et al. Molecular Effects of Glycerol on Lipid Monolayers at the Gas–Liquid Interface: Impact on Microbubble Physical and Mechanical Properties. Langmuir. 2019;35:10097–105. [DOI] [PubMed] [Google Scholar]
- [44].Ward JP, Feldman DS, Paul J, Sala DA, Errico TJ, Otsuka NY, et al. Wound Closure in Nonidiopathic Scoliosis: Does Closure Matter? Journal of Pediatric Orthopaedics. 2017;37. [DOI] [PubMed] [Google Scholar]