Figure 1.
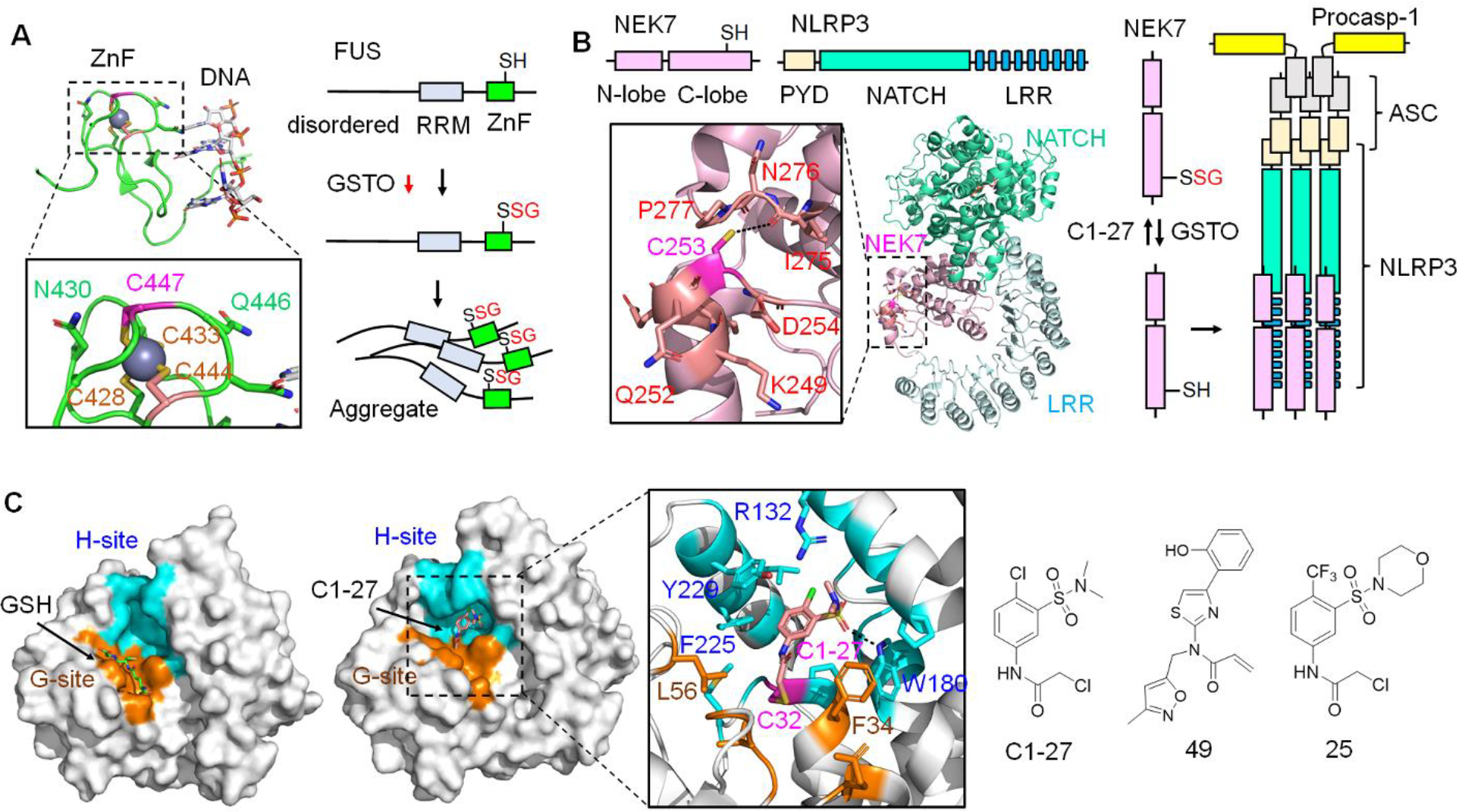
GSTO1 substrates and Inhibitors. A. GSTO1 regulates FUS SSG associated with amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD). FUS is susceptible to SSG at C447 (bound to zinc) in the zinc finger domain (ZnF, PDB: 6G99). FUS SSG increases its aggregation, causing toxicity in motor neurons linked to neurodegeneration. GSTO1 reduces FUS SSG and protects from FUS-induced toxicity. B. GSTO1 activates NLRP3 inflammasome via NEK7 deglutathionylation. NEK7 was found glutathionylated at C253 (kinase C-lobe, PDB: 6NPY) in macrophages. GSTO1 binds to NEK7, causing its deglutathionylation. NEK7 deglutathionylation enables its interaction with NLRP3, inducing NLRP3 inflammasome complex formation with ASC and pro-caspase-1. The NLRP3 inflammasome activates caspase-1, which activates inflammatory cytokines (IL-1β and IL-18). C1–27 inhibits GSTO1, thus increasing NEK7 SSG and reducing NLRP3 inflammasome-mediated IL-1β release. C. GSTO1 inhibitors. GSTO1 has reactive cysteine (C32) with a GSH binding site (G-site, orange) and a hydrophobic site (H-site, cyan) (PDB: 1EEM). C1–27 inhibits GSTO1 by primarily binding at the H-site (cyan) while covalently conjugated to C32 (PDB: 4YQM). Dotted lines indicate a distance less than 4 Å. C1–27 was used as a lead compound to develop GSTO1 inhibitors, acrylamide-containing compound 49 with the highest potency (IC50 = 0.22 nM) and α-chloroacetamide derivative 25 with improved microsomal stability (half-life = 1.4 h).
