Abstract
Ferroptosis is an iron-dependent and lipid peroxidation-driven cell death cascade, occurring when there is an imbalance of redox homeostasis in the cell. Nuclear factor erythroid 2-related factor 2 (NFE2L2, also known as NRF2) is key for cellular antioxidant responses, which promotes downstream genes transcription by binding to their antioxidant response elements (AREs). Numerous studies suggest that NRF2 assumes an extremely important role in the regulation of ferroptosis, for its various functions in iron, lipid, and amino acid metabolism, and so on. Many pathological states are relevant to ferroptosis. Abnormal suppression of ferroptosis is found in many cases of cancer, promoting their progression and metastasis. While during tissue damages, ferroptosis is recurrently promoted, resulting in a large number of cell deaths and even dysfunctions of the corresponding organs. Therefore, targeting NRF2-related signaling pathways, to induce or inhibit ferroptosis, has become a great potential therapy for combating cancers, as well as preventing neurodegenerative and ischemic diseases. In this review, a brief overview of the research process of ferroptosis over the past decade will be presented. In particular, the mechanisms of ferroptosis and a focus on the regulation of ferroptosis by NRF2 will be discussed. Finally, the review will briefly list some clinical applications of targeting the NRF2 signaling pathway in the treatment of diseases.
Keywords: NRF2, ferroptosis, antioxidant, metabolism
1. Introduction
Ferroptosis was first observed in 2003 by Dixon, in whose observation, cancer cells with RAS mutations were selectively eliminated by erastin, which was newly discovered by Dolma and his colleague [1]. Subsequently, Yagoda [2] and Yang [3] in 2007 and 2008, respectively, found that iron chelators could inhibit this kind of cell death and discovered another compound, RSL3 (RAS synthetic lethal 3), which could activate it. In 2012, when studying the mechanism behind erastin killing cancer cells, Dixon et al. officially coined this mode of death as ferroptosis based on its unique features [4].
The morphological, biochemical, and genetic characteristics of ferroptosis are distinct from other types of cell death [4]. Morphologically, ferroptosis does not perform typical features of necrosis (such as plasma membrane breakdown and generalized swelling of the cytoplasm and organelles), neither does it have the properties of apoptosis (such as cellular and nuclear volume reduction, nuclear fragmentation, and formation of apoptotic bodies). The main recognizable ultrastructural characteristic of ferroptosis is the apparently altered mitochondrial morphology. Earlier studies showed that mitochondria in ferroptosis cells were significantly contracted along with increased membrane density [2,4]. Later studies illustrated that ferroptosis caused mitochondrial swelling, reduced cristae, and caused outer membrane rupture [5,6,7,8]. Biochemically, there are prominent features that can distinguish ferroptosis from others. For example, intracellular depletion of GSH (glutathione), decreased activity of GPX4 (glutathione peroxidase 4), and accumulation of lipid peroxides are regarded as essential factors to promote ferroptosis [3,5]. In genetics, ferroptosis is a biological process regulated by multiple genes, involving regulatory mechanisms in iron homeostasis, lipid peroxidation metabolism, amino acid metabolism, and so on.
Just as its name implies, NRF2 (nuclear factor erythroid 2-related factor 2, NFE2L2) originated from the study of erythropoiesis. In 1994, Moi et al. [9] identified NRF2 as one of the DNA-binding proteins that could bind to AP-1/NF-E2 (activating protein-1/nuclear factor erythroid 2) in the locus control region of β-globin, assisting NF-E2 to mediate globin gene expression. Subsequently, in 1997, pioneering work by Itoh and his colleague demonstrated that NRF2 acted an essential part in the transcriptional induction of phase II detoxification genes carrying ARE (antioxidant response elements), after which roles of NRF2 in erythrocyte generation were marginalized, with studies shifting their focus from erythropoiesis to toxicology [10].
Most importantly, so far, almost all genes that participate in ferroptosis are involved in the transcriptional regulation of NRF2, including glutathione regulation (such as System Xc− and GPX4), iron regulation (such as FTH1 (ferritin heavy chain 1), FTL (ferritin light chain) and FPN1 (ferrous iron exporter ferroportin 1)), NADPH regeneration (such as G6PD (glucose-6-phosphate dehydrogenase), and ME1 (malic enzyme 1)), and so on [11,12,13,14], which are described in this review too. In addition, studies have suggested that NRF2 indirectly participated in the regulation of lipid metabolism via PPARγ (peroxisome proliferator-activated receptor-γ) whose abundance was associated with cell sensitivity to ferroptosis [6]. Hence, with abundant upstream and downstream effectors, targeting NRF2 signaling pathway can effectively affect ferroptosis. In other words, therapies that promote or inhibit ferroptosis through modulating NRF2 signaling pathway are feasible and are worth the time and effort required for their exploration.
2. Ferroptosis
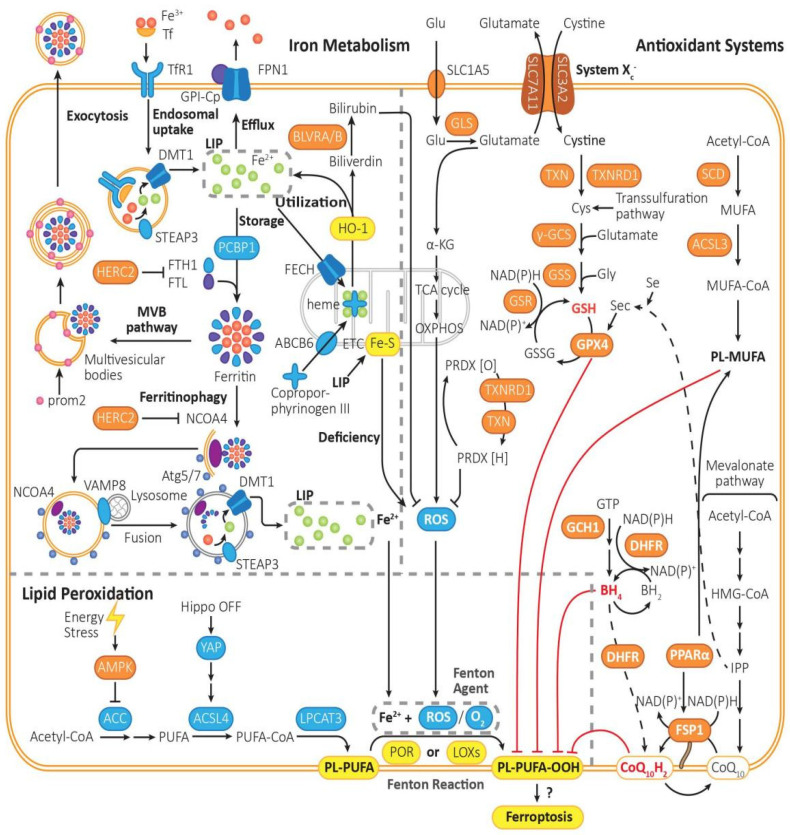
The key process of ferroptosis is Fenton reaction—Fe2+ and O2 (or ROS, reactive oxygen species) function as strong oxidants and peroxidize PL-PUFA (phospholipid with polyunsaturated fatty acyl tail) to PL-PUFA-OOH [15] in the catalysis of POR (cytochrome p450 oxidoreductases) [16] or LOXs (lipoxygenases) [17]. PL-PUFA-OOH then triggers the followed reactions of ferroptosis (Figure 1), though the mechanism of which is still not quite clear.
Figure 1.
The mechanism of ferroptosis. Ferroptosis is driven by iron-dependent lipid peroxidation, the reaction of PL-PUFA with Fe2+ and ROS under the catalysis of POR or LOXs. Ferroptosis is regulated by lipid metabolism, iron metabolism, and intracellular antioxidant system. (1) Lipid metabolism: PL-PUFA, the prime substrate for lipid peroxidation, is synthesized from Acetyl-CoA in multiple steps, which are catalyzed by ACC, ACSL4, and LPCAT3. Then, it is prone to be peroxidized to PL-PUFA-OOH, which triggers the followed reactions of ferroptosis, though the initiation mechanism is still not quite clear. The peroxidation process is mainly driven by Fenton reaction, or mediated by POR or LOXs. By activating AMPK, energy stress can inhibit the activity of ACC, thus turning on an energy stress-protective program against ferroptosis. (2) Iron metabolism: The peroxidation of PL-PUFA is driven by the labile iron pool and iron-dependent enzymes. Fe3+ is imported into cells by Tf through TfR1, then being reduced by STEAP3 to Fe2+, forming the labile iron pool. Excess Fe2+ can be expelled via FPN1, be stored in ferritin, which can be exported through MVB pathway or release Fe2+ through ferritinophagy, or be utilized to synthesize heme with coproporphyrinogen III through ABCB6 and FECH, or regenerated when heme is metabolized by HO-1. (3) Intracellular antioxidant system: There are mainly three pathways for removing PL-PUFA-OOHs. Cyst(e)ine/GSH/GPX4 axis: It is one of the most important pathways in ferroptosis inhibition. It requires uptake of cystine via system Xc−, reduction of cystine to cysteine, and biosynthesis of GSH. GPX4-mediated reduction of PL-PUFA-OOH to PL-PUFA-OH, and regeneration of GSH from GSSG. FSP1/CoQ10 axis: It functions independently of GPX4. CoQ10H2 can remove lipid peroxides, and then consume NADPH to regenerate CoQ10H2 by FSP1. GCH1/BH4/DHFR system: BH4 can be synthesized from GTP under the catalysis of GCH1, being converted into BH2 and clearing lipid peroxidants, and then regenerated by DHFR. Furthermore, saturated fatty acid can be transformed into PL-MUFA in multi-steps, which is the competing substrate against PL-PUFA and can suppress ferroptosis. Furthermore, PRDX can reduce the content of ROS, which can be generated from PRDX[O] through NADPH and TRX.
2.1. Processes That Promote Ferroptosis
Other than PE (phosphatidylethanolamines) [18], PUFAs (polyunsaturated fatty acids) might be the prime substrates for lipid peroxidation according to recent data [19]. PL-PUFAs are sensitive to peroxidation because of their C=C bond. PL-PUFAs are biosynthesized from acyl-CoA in multi-steps in the catalysis of ACC (acetyl coenzyme A carboxylase) [20,21], ACSL4 (acyl-CoA synthetase long-chain family member 4) [22], and LPCAT3 (lysophosphatidylcholine acyltransferase 3) [23]. PUFAs must be esterified into membrane PL-PUFAs so that they can be peroxidized to transmit the ferroptosis signal.
Energy stress (such as glucose starvation) inhibits lipogenesis and ferroptosis through activation of AMPK (adenosine-monophosphate-activated protein kinase), which inhibits the activity of ACC and biosynthesis of PUFAs [17]. E-cadherin-mediated cell–cell contacts activate Hippo signaling and thus inhibit nuclear translocation of NRF2 and the activity of transcriptional co-regulator YAP (Yes-associated protein), which promote transcription of ACSL4, TfR1 (transferrin receptor 1), and possibly other contributors of ferroptosis. Thus, closure of Hippo pathway and increased YAPs can make cells more sensitive to ferroptosis [24].
2.1.1. LIP (Liable Iron Pool): Iron Drives Lipid Peroxidation with the Aid of ROS
Fe3+ formed from intestinal absorption or erythrocyte degradation can be captured by Tf and imported into cells through TfR1 [25], after which it is reduced to Fe2+ by STEAP3 (six transmembrane epithelial antigen of the prostate 3) [26] and imported via DMT1 (ferrous ion membrane transport protein) [27]. Fe2+ is maintained in the LIP, bound to low molecular weight compounds, including GSH (glutathione) [28].
The excess Fe2+ can be oxidized and then exported by FPN1 (ferrous iron exporter ferroportin 1) in the plasma membrane [29], utilized by mitochondria for heme synthesis, or oxidized and stored in ferritins by PCBP1 (poly-(rC)-binding protein 1) [30]. Heme synthesis in the mitochondria is regulated by ABCB6 (ATP-binding cassette subfamily B member 6) and FECH (ferrochelatase), which, respectively, transport coproporphyrinogen III and Fe2+ from the cytosol to the mitochondrial intermembrane space to form heme [31]. For heme degradation, HO-1 (heme-oxygenase 1) breaks down heme into biliverdin and liable Fe2+. Biliverdin is further metabolized to a potent antioxidant called bilirubin by BLVRA/BLVRB (biliverdin reductase A and biliverdin reductase B) [32,33]. Thus, HO-1 may play a dual role in ferroptosis. Some studies suggested that HO-1 promotes ferroptosis [34,35], while others reported opposing results that knockout or knockdown of HO-1 exacerbates ferroptosis [36,37,38].
Ferritins can be exported through MVBs (prom2-mediated ferritin-containing multivesicular bodies) when necessary [39], while some can be degraded into liable Fe2+ through ferritinophagy triggered by NCOA4 (nuclear receptor coactivator 4) [40]. In ferritinophagy, NCOA4 recruits ferritins into autophagosome, and then VAMP8 (vesicle associated membrane protein 8) mediates autophagosome–lysosome fusion [40], after which newly freed Fe3+ can be reduced and transported into cytosol LIP by STEAP3 and DMT1 [41]. Except NCOA4, there are other ferritinophagy activation proteins, such as ULK1 (unc-51 like kinase 1), atg5 (recombinant autophagy-related protein 5), and atg7 (recombinant autophagy-related protein 7) [42].
2.1.2. Run-Out Mitochondrial Respiration Boosts ROS Generation
The out-of-control respiration of mitochondria is assumed to act as a promoting effect on ferroptosis because of the generation of more ROS [43]. Glutaminolysis is assumed to be an important factor for the run-out mitochondrial respiration. Glutamate is a precursor of α-KG (α-ketoglutaric acid), and hence, glutamate converted into α-KG accelerates the TCA (tricarboxylic acid) cycle and mitochondrial ROS generation (with hyperpolarization of inner mitochondrial membrane potential) [44].
2.2. Processes That Inhibit Ferroptosis
2.2.1. Cyst(e)ine/GSH/GPX4 System
Cysteine and glutamate are essential for GSH biosynthesis to protect against oxidation. Cystine is imported by system Xc−, reduced into cysteine by TXN/TXNRD1 or through transsulfuration pathway [45] (reductive power from NAD(P)H), and utilized in GSH synthesis in the catalysis of GCL (glutamate-cysteine ligase, or γ-GCS, γ-glutamylcysteine synthetase) and GSS (glutathione synthetase). GPX4 (glutathione peroxidase 4) converts GSH into GSSG (oxidized glutathione) and reduces L-OOH (lipid peroxides) to the corresponding L-OH (lipid alcohols) [46]. TXN (thioredoxin) and TXNRD1 (thioredoxin reductase) contribute to the reduction of cystine to cysteine [47,48].
System Xc− is an amino acid anti-porter in membrane, composed of two subunits, SLC7A11 (xCT) and SLC3A2, which imports cystine and exports glutamate in a ratio of 1:1 [4]. And SLC7A11 can be inhibited by p53 [49]. Since GPX4 is a selenoprotein, its biosynthesis relies on the co-translational incorporation of Sec (selenocysteine) [50].
2.2.2. FSP1/CoQ10/NAD(P)H System
FSP1 (ferroptosis suppressor protein 1) can convert CoQ10 (ubiquinone) to CoQ10H2 (ubiquinol) with NAD(P)H, adding to the antioxidation capacity [51]. The intermediate of CoQ10, IPP (isopentenyl pyrophosphoric acid, or isopentenyl-PP), is as important as CoQ10 for the maturation of selenocysteine tRNA for GPX4 [52]. FSP1 can be activated by PPARα (peroxisome proliferator-activated receptor-α) [53].
2.2.3. GCH1/BH4/DHFR System
GCH1 (GTP cyclohydrolase 1) is the rate-limiting enzyme for BH4 (tetrahydrobiopterin) synthesis. BH4 can reduce lipid peroxidants and be converted into BH2 (dihydrobiopterin), which can be regenerated from BH2 with NAD(P)H by DHFR (dihydrofolate reductase). BH4 can also promote the synthesis of CoQ10 by DHFR [54].
2.2.4. Lipid Metabolism: PL-MUFA Inhibits Peroxidation of PL-PUFA
MUFA (monounsaturated fatty acid) biosynthesis requires SCD (stearoyl-CoA desaturase), which converts saturated fatty acids into MUFAs [55]. MUFA can be converted into MUFA-CoA by ACSL3 (acyl coenzyme A synthetase long-chain family member 3) and ultimately forms PL-MUFA (phospholipid with monounsaturated fatty acyl tail), the competing substrate against PL-PUFA [56]. Thus, the biosynthesis of PL-MUFA inhibits ferroptosis.
3. NRF2 Structure and Function
3.1. NRF2 Structure
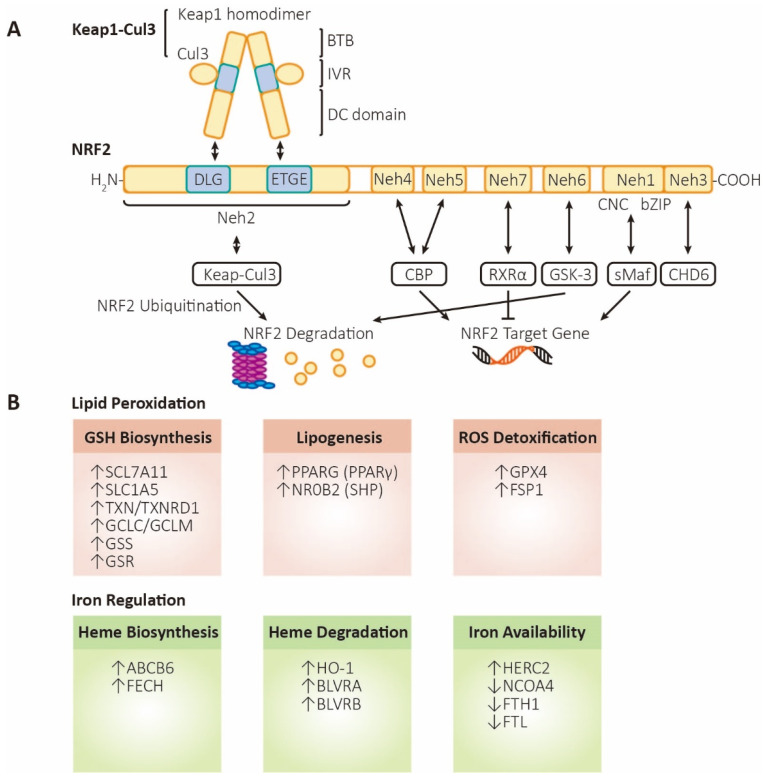
NRF2 (nuclear factor erythroid 2-related factor 2, also named NFE2L2) contains 605 amino acids with seven highly conserved homology domains, named Neh1 (NRF2-ECH homology domain-1) to Neh7 (Figure 2). The Neh1 domain contains CNC/bZIP (Cap-N-Collar/basic leucine zipper) motifs, which can be bound with sMAF (small musculoaponeurotic fibrosarcoma) to form heterodimers that bind to AREs [57,58]. The amino terminal domain or carboxyl terminal domain is named Neh2 or Neh3, individually. The DLG and ETGE motifs on Neh2 domain bind with DC domain of Keap1 (kelch-like ECH-ssociated protein 1) homodimer [44,59], leading to the ubiquitination and degradation of NRF2 [60]. The Neh3 domain includes a VFLVPK motif, which is a critical binding site for CHD6 (chromodomain helicase DNA binding protein 6) [61,62]. Neh4 and Neh5 are acidic conserved sequences, which enhance NRF2 downstream genes expression via binding CBP (cAMP responsive element binding protein) [63,64]. Neh6, a serine-rich conserved sequence, could be phosphorylated by GSK-3β (glycogen synthase kinase 3β), leading to the degradation of NRF2 [63]. The Neh6 domain likely contains another phosphorylation-dependent motif, the DSAPGS motif, an extra binding site for β-TrCP (β-transducin repeat-containing protein) [65]. The Neh7 functions as a binding site for RXRα, capable of inhibiting NRF2 and its AREs [66,67].
Figure 2.
The structure and functions of NRF2. (A) Structure and regulation of NRF2. Keap1, kelch-like ECH associated protein 1; Cul3, cullin3 E3 ubiquitin ligase; BTB, broad complex, tramtrack, and bric-a-brac domain; IVR, intermediate region; DC domain, DGR (double glycine repeat) and CTR (carboxyl terminal region) domain; NRF2, nuclear factor erythroid 2-related factor 2; Neh1~6, NRF2-ECH homology domain-1~6; CNC, Cap-N-Collar motif; bZIP, basic leucine zipper motif; sMaf, small musculoaponeurotic fibrosarcoma; CHD6, chromodomain helicase DNA binding protein 6; CBP, cAMP responsive element binding protein; GSK-3, glycogen synthase kinase 3; RXRα, retinoid X receptor alpha. (B) Functions of NRF2. Lipid peroxidation (labeled in orange, referring to processes that inhibit ferroptosis): SLC7A11 (xCT), solute carrier family 7 member 11, a heteromeric, sodium-independent, highly specific cysteine-glutamate transport system; SLC1A5, solute carrier family 1 member 5, a sodium-dependent neutral amino acid transporter; TXN, thioredoxin; TXNRD1, thioredoxin reductase 1; GCLC/GCLM, glutamate-cysteine ligase catalytic/modifier subunit, comprising heterodimer γ-GCS (gamma-glutamylcysteine synthetase); GSS, glutathione synthetase; GSR, glutathione-disulfide reductase; PPARG (PPARγ), peroxisome proliferator-activated receptor gamma; NR0B2 (SHP), nuclear receptor subfamily 0 group B member 2 (small heterodimer partner); GPX4, glutathione peroxidase 4; FSP1 (AIFM2), ferroptosis-suppressor-protein 1 (apoptosis inducing factor mitochondria associated 2). Iron regulation (labeled in green, referring to processes that promote ferroptosis): ABCB6, ATP-binding cassette subfamily B member 6, a member of the heavy metal importer subfamily, playing a role in porphyrin transport; FECH, ferrochelatase; HO-1, heme oxygenase 1; BLVRA/BLVRB, biliverdin reductase A/B; HERC2, HECT, and RLD domain containing E3 ubiquitin protein ligase 2; NCOA4, nuclear receptor coactivator 4, androgen receptor coactivator; FTH1, ferritin heavy chain 1; FTL, ferritin light chain.
3.2. NRF2 Regulation
The major approach to change NRF2 activity is regulating the stability of proteins, of which the Keap1-Cul3-Rbx1 axis is the most important regulator. In addition, NRF2 activity can also be regulated at the transcriptional and post-transcriptional levels.
As for the stability of proteins, Keap1 is considered to play a crucial role in the regulation of NRF2 stability. Therefore, NRF2 stability regulation is artificially divided into Keap1-dependent regulation and Keap1-independent regulation. As mentioned above, under normal circumstances, two molecules of Keap1 bind to the ETGE and DLG motifs on the Neh2 domain of NRF2 via its Kelch repeat domain [44,59]. As an adaptor protein for the Cul3 (Cullin3) E3 ubiquitin ligase complex, it can assemble with Cul3 and Rbx1 into a functional E3 ubiquitin ligase (Keap1-Cul3-E3), which leads to continuous ubiquitination and degradation of NRF2, ensuring low levels of intracellular NRF2 [59]. However, when cells are subjected to internal and external stimuli such as oxidative stress, Keap1 is inactivated due to modification of cysteine residues [68], competitive binding of p62 [69], and so on, causing NRF2 to dissociate from Keap1, and it becomes stable and subsequently transfers to the nucleus. Within the nucleus, NRF2 increases the transcription of antioxidant genes by interacting with other protein factors (such as sMaf) and binding to the ARE.
There are two main ways to regulate NRF2 stability independent of Keap1. One is the β-TrCP-Cul1-dependent pathway. Under the condition of homeostasis, NRF2 is phosphorylated by GSK-3β, after which it can be recognized by β-TrCP, resulting in the ubiquitination of NRF2 by the β-TrCP-Cul1 E3 ubiquitin ligase complex and later degradation by proteasome [63,70,71]. Under stress, the PI3K (phosphoinositide 3-kinase)/Akt pathway is activated, leading to the suppression of GSK-3β by phosphorylation, thus activating NRF2 [72]. The other is the Hrd1-dependent pathway. Hrd1 is an E3 ubiquitin ligase that binds directly to NRF2 and causes the ubiquitination and degradation of NRF2, negatively regulating NRF2 protein levels [73].
As for the regulation at the transcriptional level, Hrd1 is mainly involved in some transcriptional factors that can bind to specific sites in the promoter of NRF2 gene, such as AhR (aryl hydrocarbon receptor) [74], NF-κB (nuclear factor-κB) [75], NRF2 [76], and so on, which can increase the transcription of NRF2. Regulation of NRF2 also occurs at the post-transcriptional level. For example, NRF2 is regulated by a variety of miRNA genes, some of which (such as miR-144) can be locally complementary to the mRNA of NRF2 to silence the NRF2 gene [77]. In addition, in some tumor cells, the mRNA precursor of NRF2 can undergo alternative splicing, resulting in loss of the domain interacting with Keap1, so that Keap1 fails to bind to NRF2 normally, leading to NRF2 stabilization and a decrease in ferroptosis in cancer cells [78].
3.3. NRF2 Functions
3.3.1. Antioxidant-Dependent Function
-
(1).
Glutathione metabolism
Two crucial enzymes in GSH biosynthesis are regulated by NRF2—GCL (composed of GCLC/GCLM, glutamate-cysteine ligase catalytic/modulatory subunits) and GSS [11,76,79,80,81]. SLC7A11, one of the two subunits of system Xc−, is also regulated by NRF2 in GSH metabolism, which is responsible for transporting cystine into the cell, thereby increasing cysteine content and promoting the process of GSH generation [80,81].
With the assistance of GSH, GPX4 exerts the activity of reducing peroxides. GSH serves as a reducing agent that donates electrons to xenobiotics or electrophilic organisms, thus transforming into its oxidized form GSSG. Research has shown that GPX4 mainly acts on H2O2 or organic hydrogen peroxide compounds, as well as ONOO- [82]. The resulting agent, GSSG, is subsequently reduced for the maintenance of intracellular GSH pool. This process is completed by GSR (glutathione-disulfide reductase) with NADPH [83]. According to reports, both GPX4 and GR are found being transcriptionally regulated by NRF2 [84,85].
-
(2).
FSP1-CoQ10 axis
FSP1 is a lipophilic antioxidant, which has been confirmed to be targeted by NRF2. The enzyme regenerates ubiquinol from ubiquinone, suppressing the formation of PL-PUFA-OOHs [19,86]. By regulating the expression level of FSP1, NRF2 maintains low levels of cellular peroxides.
-
(3).
Components of the erythrotoxin family
Besides GSH, another system that resists oxidative stress is the erythrotoxin system. PRDX (peroxiredoxin) can reduce ROS level and is oxidized during this process. Oxidized PRDX is then reduced by TXN (thioredoxin), with the participation of cysteine oxidized by ROS. TXNRD1 (thioredoxin reductase 1) is used to rescue oxidized TXN with NADPH [87,88]. The expression of PRDX, TXN, and TXNRD1 mentioned above are all controlled by NRF2 at transcription level [89].
3.3.2. Antioxidant-Independent Function
NRF2 can exert its antioxidant-independent effects by orchestrating the genes expression in phase II detoxification processes [90]. It activates the transcription of genes encoding detoxification enzymes such as GSTs (glutathione S-transferases) and NQO1 (NAD(P)H:quinone oxidoreductase 1) [90]. These enzymes play pivotal roles in cellular detoxification, facilitating the conjugation and elimination of harmful substances and xenobiotics. In the realm of inflammation, NRF2 activation demonstrates anti-inflammatory properties by modulating the expression of genes involved in inflammatory signaling pathways [91]. In recent years, NRF2 has been found to play a critical role in cellular metabolism.
-
(1).
Heme/iron metabolism
As an E3 ubiquitin ligase, HERC2 is responsible for the degradation of NCOA4 (ferritin cargo receptor mediating ferritinophagy) and FBXL5 (IRP1/2 regulator mediating ferritin synthesis) and thus downregulates liable iron level. HERC2 is upregulated by NRF2 to fight against ferroptosis [40]. FTL and FTH1, light and heavy chains of ferritin, respectively, are controlled by NRF2. FTL is the main stabilizer of ferritin, and FTH1 contains active sites of iron oxidase, through which Fe2+ is oxidized into Fe3+ and is stored in the central core. FPN1/SLC40A1 responsible for iron excretion from cells is also controlled by NRF2. In addition, HMOX1/HO-1, ABCB6, FECH, SLC48A1 (member A1 of lipolytic vector family 48), and BLVRA/B are also positively regulated by NRF2 [31,32,92,93,94]. Among them, SLC48A1 is responsible for transporting the released heme from the lysosome to the cytoplasm after the degradation of hemoglobin in aged red blood cells, reducing the degradation of heme.
-
(2).
NADPH generation in glucose metabolism
NADPH generation occurs in many pathways such as the PPP (pentose phosphate pathway), NADK (NAD kinase)-catalyzed phosphorylation of NADH, and the transformation of isocitrate to α-KG by IDH (NADP-dependent isocitrate dehydrogenase). NRF2 directly regulates the transcription of various PPP enzymes, such as G6PD (glucose-6-phosphate dehydrogenase) and other oxidative PPP enzymes [95], comprising reversible and irreversible reactions [96]. NRF2 transcriptionally regulates ME1 (malic enzyme 1) and IDH1 (isocitrate dehydrogenase 1) as well, contributing to the generation of NADPH [95]. NADPH is not only crucial for the synthesis of fatty acids (such as PUFAs), cholesterol, and nucleotides [97], but also important in antioxidation axes such as GPX4, FSP1, and DHFR [19,98,99].
-
(3).
Lipid metabolism
Lipid synthesis highly consumes NADPH and competes with cellular antioxidant systems. Studies have shown that activation of NRF2 mitigates the generation and desaturation of fatty acid [13,54] to increase NADPH content for detoxification in murine liver.
PPARγ (peroxisome proliferator-activated receptor gamma) and NR0B2 (nuclear receptor subfamily 0, group B, member 2) are nuclear receptors that play significant parts in the regulation and control of cholesterol metabolism, which may aid in anti-peroxidation. Both genes can be activated by NRF2 and thus play a crucial role in mitigating accumulation of lipid peroxides [93,100,101].
-
(4).
Amino acid metabolism
NRF2 positively regulates xCT (SLC7A11, the subunit of system Xc−), which imports cystine and exports glutamate [102]. NRF2 also regulates TXN and TXNRD1 at transcriptional level, both of which help cystine to be reduced to cysteine [47,48]. SLC1A5 (solute-linked carrier family A1 member 5), which can be activated by NRF2, plays an essential role in glutamine uptake, which contributes to GSH biosynthesis [103]. But some studies showed that NRF2-actived cells are in a state of glutamate deficiency due to elevated xCT, GCLC/GCLM, and GSS. As a result, less α-KG is generated from glutamate and TCA cycle is restricted, hence inhibiting generation of ROS by ETC (electron transport chain), the downstream process of TCA cycle [104].
These antioxidant-independent functions of NRF2 mentioned above illustrate its multifaceted role in maintaining cellular homeostasis and influencing disease pathogenesis. Elucidating the intricate mechanisms underlying these functions holds promise for the development of innovative therapeutic approaches by targeting NRF2 activation in various pathological conditions associated with inflammation, metabolic disorders, and tissue damages.
4. NRF2 in Ferroptosis
NRF2 is kept low by three E3 ubiquitin ligases (KEAP1-CUL3-RBX1, SCF/βTrCP, and synoviolin/Hrd1). In the presence of gene mutation, endogenously induced modification, competitive binding of other interacting particles, and inhibition of exogenous drugs, NRF2 will be activated and transferred into the nucleus to initiate the transcription of target genes (Table 1).
Table 1.
Major roles of NRF2 in ferroptosis (via regulating downstream genes).
| Symbol | Whole Name | Function | Upregulation or Downregulation | Main Factors for Inhibiting Ferroptosis |
|---|---|---|---|---|
| ABCB6 | ATP-binding cassette subfamily B member 6 | Heme synthesis (transport protoporphyrin to mitochondria) |
↑upregulation | Decrease the capacity of LIP |
| FECH | Ferrochelatase | Heme synthesis (insert ferrous iron into protoporphyrin) |
↑upregulation | Decrease the capacity of LIP |
| FTH1 | Ferritin heavy chain 1 | Iron storage (oxidize ferrous iron into ferric iron) |
↓downregulation | Decrease the capacity of LIP |
| FTL | Ferritin light chain | Iron storage (stabilize ferritin) |
↓downregulation | Decrease the capacity of LIP |
| FPN1 | Ferroportin 1 | Iron excretion | ↑upregulation | Decrease the capacity of LIP |
| HO-1 | Heme-oxygenase 1 | Heme degradation (decompose heme into ferrous iron and biliverdin) |
↑upregulation | Increase the capacity of LIP but generate antioxidants (HO-1 exerts dual effects on ferroptosis) |
| BLVRA/B | Biliverdin reductase A/B | Heme degradation (metabolize biliverdin into bilirubin) |
↑upregulation | Generate antioxidant: bilirubin |
| HERC2 | HECT and RLD domain containing E3 ubiquitin protein ligase 2 | Inhibit ferritinophagy (NCOA4 and FBXL5 ubiquitination and degradation) | ↑upregulation | Decrease the capacity of LIP |
| SLC7A11 | Solute carrier family 7 member 11 | GSH synthesis (import cystine and export glutamate) |
↑upregulation | Generate antioxidant: GSH |
| SLC1A5 | Solute carrier family 1 member 5 | GSH synthesis (absorb glutamine) |
↑upregulation | Generate antioxidant: GSH |
| TXN/TXNRD1 | Thioredoxin/Thioredoxin reductase 1 | GSH synthesis (reduce cystine to cysteine) |
↑upregulation | Generate antioxidant: GSH |
| GCLC/GCLM | Glutamate-cysteine ligase catalytic/modifier subunits | GSH synthesis (connect glutamate and cysteine) |
↑upregulation | Generate antioxidant: GSH |
| GSS | Glutathione synthetase | GSH synthesis (connect glycine and γ-glutamyl cysteine) |
↑upregulation | Generate antioxidant: GSH |
| GSR | Glutathione-disulfide reductase | GSH synthesis (reduce GSSG to GSH) |
↑upregulation | Generate antioxidant: GSH |
| GPX4 | Glutathione peroxidase 4 | ROS detoxification (reduce lipid peroxides by oxidizing GSH to GSSG) |
↑upregulation | Decrease the accumulation of lipid peroxides |
| FSP1 | Ferroptosis-suppressor-protein 1 | Ubiquinol synthesis (convert ubiquinone to ubiquinol with NAD(P)H) |
↑upregulation | Generate antioxidant: ubiquinol |
| PPARγ | Peroxisome proliferator-activated receptor gamma | ROS detoxification (mitigate lipid peroxidation as a nuclear receptor) |
↑upregulation | Decrease the accumulation of lipid peroxides |
| NR0B2 | Nuclear receptor subfamily 0, group B, member 2 | ROS detoxification (mitigate lipid peroxidation as a nuclear receptor) |
↑upregulation | Decrease the accumulation of lipid peroxides |
4.1. Target NRF2 Signaling Pathway and Promote Ferroptosis in Cancer Therapy
Ferroptosis has emerged as a subject of significant interest within the cancer research field. Its distinct mechanistic and morphological attributes differentiate ferroptosis from other cell death modalities, thereby rendering it a promising avenue for cancer therapeutic exploration. The inhibition of ferroptosis observed across various cancer types, coupled with its dynamic role as a tumor suppressor during cancer progression, suggests that manipulating the regulation of ferroptosis holds promise as an interventional strategy in tumor treatment [105]. Consequently, small molecules that reprogram cancer cells to undergo ferroptosis are regarded as potent therapeutic agents for cancer therapy. Ferroptosis inducers can be classified into several different groups, each acting through different mechanisms.
4.1.1. Four Types of FIN (Ferroptosis Inducers)
-
(1).
Class I FIN: system Xc− inhibitors
System Xc− inhibitors, including erastin, can block uptake of cystine and reduce cysteine content, leading to a significant reduction in GSH levels, lipid ROS-mediated damage, and finally ferroptosis [46]. Biochemical and metabolomic analyses have demonstrated a notable decrease in GSH levels following erastin treatment [106]. Studies have also observed decreased GPX4 activity in various cancer cells treated with erastin [107]. Moreover, AUR (auranofin) is able to trigger ferroptosis in liver by inhibiting the activity of TXNRD and facilitating lipid peroxidation when given a high dose while a lower dose might be relatively safe [108]. Similar to erastin, SAS (sulfasalazine) can inhibit system XC− and then trigger ferroptosis [109].
-
(2).
Class II FIN: glutathione peroxidase 4 (GPX4) inhibitors
The conventional inducer of ferroptosis, RSL3 ((1S,3R)-RSL3), functions by covalently binding to the active site seleno-cysteine residue of the glutathione peroxidase 4 (GPX4) enzyme, irreversibly inhibiting its activity. This inhibition or loss of GPX4 activity results in the accumulation of lipid peroxides, which are considered lethal signals for ferroptosis. Different from RSL3, FIN56, derived from CIL56, can induce degradation of GPX4 with the participation of ACC and activate SQS which leads to CoQ10 depletion, rather than directly inhibit GPX4 activity [21]. Furthermore, exhaustion of GSH as well as the inactivation of GPX4 may play an important role in cisplatin-induced ferroptosis and apoptosis in A549 and HCT116 cells [110].
-
(3).
Class III FIN: ubiquinone generation inhibitors
Statins, including fluvastatin, lovastatin, and simvastatin, are a class of pharmaceutical agents utilized for the reduction of blood cholesterol levels [111]. These drugs exert their cholesterol-lowering effects by inhibiting HMGCR (3-hydroxy-3-methylglutaryl-coenzyme A reductase), an enzyme responsible for the rate-limiting step in the mevalonate pathway, which is involved in cholesterol synthesis. By impeding the generation of isopentenyl pyrophosphate, an intermediate metabolite in the mevalonate pathway, statins can also hinder the biosynthesis of selenoproteins, including GPX4, and CoQ10, hence enhance ferroptosis [21]. The established clinical safety profile of statins, coupled with obesity being a significant risk factor for cancer, has led to the initiation of numerous clinical trials investigating the efficacy of statins as monotherapy or combination therapy in various types of tumors [112].
-
(4).
Class IV FIN: lipid metabolism disruptors
Excessive iron levels induce the generation of reactive oxygen species (ROS), leading to an escalation in lipid peroxide levels. This lipid peroxide accumulation within cell membranes serves as a source of lipid ROS, which further contributes to the initiation of ferroptosis [2,3,4].
FINO2 (an endoperoxide containing 1,2-dioxolane) can directly oxidizes iron, leading to widespread lipid peroxidation via Fenton reaction and finally ferroptosis ultimately [113]. Lapatinib is able to attenuate the expression of ferroportin and ferritin and promotes the expression of transferrin, causing an increase in intracellular iron ion levels and subsequently ferroptosis [114]. Studies have shown that artemisinin derivatives induce ferroptosis in tumor cells by oxidizing iron, generating more ROS and fostering Fenton reaction [115].
4.1.2. Clinical Application of Targeting NRF2 and Ferroptosis in Cancer Therapy
NRF2 can fight against ferroptosis by regulating its downstream antioxidant genes, but this also means that it maintains the survival of tumor cells while protecting normal cells. In the past decade, many studies have revealed that the activation of NRF2 in cancer cells promotes cancer progression [116,117,118] and metastasis [119], and confers cancer cells resistance to chemotherapy and radiotherapy [120,121]. Based on the mechanism of ferroptosis inducers mentioned above, drugs have been designed and invented in order to induce ferroptosis by targeting NRF2 signaling pathway in cancer therapy, and some of which are feasible enough to be utilized and evaluated in clinical trials (Table 2). Several drugs are described in detail below, by considering some cancer types as examples.
Table 2.
NRF2 inhibitors and activators.
| Inhibitors | ||||
|---|---|---|---|---|
| Compound | Model Organism | Mechanism | Diseases | References |
| Sorafenib | HT-1080 Calu-1 et al. |
Inhibit NRF2/xCT signaling pathway | HCC | [109] |
| Metformin | HepG2, HUH-7, BALB/C nude mice |
Inhibit NRF2 nuclear translocation | HCC resistant to sorafinib | [122] |
| APG | BEL-7402,BEL-7402/ADM | Enhance NRF2 degradation (upregulate miR-101 expression) |
HCC | [123] |
| (1S,3R)-RSL3 | BJeLR et al. | Inhibit NRF2/GPX4 signaling pathway | BC | [124] |
| Fascin | HS578T MDA-MB-231 et al. |
Inhibit NRF2/xCT signaling pathway (induce xCT degradation) |
BC | [125] |
| Isoorientin | A549 | Inhibit SIRT6/NRF2/GPX4 signaling pathway | Lung cancer resistant to DDP | [126] |
| ZVI-NP | H1299, H460, A549 | Enhance NRF2 degradation (by GSK3/β-TrCP) |
Lung cancer | [127] |
| Wogonin | AsPC-1, PANC-1, HPDE6-C7 | Inhibit NRF2/GPX4 signaling pathway | Pancreatic cancer | [128] |
| Trigonelline | Athymic BALB/C male nude mice | Silence NRF2 by RNA interference | Drug-resistant head and neck cancer | [37] |
| Sulfasalazine | OSC19, HSC-2 et al. | Inhibit NRF2/xCT signaling pathway | Lymphoma, head and neck squamous cell carcinoma, gastric tumor |
[129,130] |
| Activators | ||||
| Compound | Model Organism | Mechanism | Diseases | References |
| Levistilide A | MDA-MB-231, MCF-7 | Activate NRF2/HO-1 signaling pathway | BC | [131] |
| Tagitinin C | CRC cell lines | Enhance NRF2 nuclear translocation | Colorectal cancer | [132] |
| DMF | Mouse embryonic fibroblasts (MEFs) | Enhance NRF2 stabilization (modify key cysteine residues on Keap1) |
Alzheimer’s disease | [133] |
| SIRT1 | Diabetic Sprague Dawley rat model, H9C2 |
Enhance NRF2 nuclear translocation | Myocardial ischemia-reperfusion injury | [134] |
| MiR-24-3p | C57BL/6 mice, H9C2 | Inhibit Keap1 expression Activate NRF2/HO-1 signaling pathway |
Myocardial ischemia-reperfusion injury | [135] |
| MiR-24 | Diabetic Sprague Dawley rat model VSMCs |
Activate NRF2/HO-1 signaling pathway | Diabetics (oxidative stress in VSMCs stimulated by high glucose) | [135] |
| Dexmedetomidine | Transient global cerebral ischemia rat model | Activate NRF2/HO-1 signaling pathway | Brain injury related to transient global cerebral ischemia | [136] |
| BCP | Cerebral ischemia/reperfusion SD rat model | Enhance NRF2 nuclear translocation | Ischemia brain injury | [137] |
| XJZ | C57BL/6 mice | Activate p62/Keap1/NRF2 pathway | Gastric mucosal injury | [138] |
| AS | Specific-pathogen-free (SPF) male Kunming (KM) mice | Enhance NRF2 nuclear translocation | Sepsis-induced lung injury | [139] |
| MiR-200a | Goto-Kakizaki (GK) rat model | Enhance NRF2 nuclear translocation (inhibit Keap1 translation) |
Experimental diabetic nephric injury | [140] |
-
(1).
Liver cancer
HCC (Hepatocellular carcinoma) is the primary malignant tumor in the liver, accounting for 90% of all liver tumors, and is the fourth leading cause of cancer death worldwide [141,142]. Sorafenib has been in the market for more than ten years as a first-line targeted drug for patients with advanced liver cancer. As a multi-kinase inhibitor, sorafenib targets tumors mainly by inhibiting tumor angiogenesis. Years of studies on the cytotoxicity of sorafenib in vitro have found that sorafenib induces an iron-dependent cell death different from apoptosis by inhibiting SLC7A11 [109], an element in the NRF2 downstream signaling pathway, thus blocking the transmembrane transport of cysteine and suppressing the biosynthesis of GSH. Results show that such death can be protected by inhibitors of ferroptosis such as liproxstatin-1, Fer-1 (ferrostatin-1) or DFO (deferoxamine), indicating that sorafenib can induce ferroptosis of liver cancer cells [143]. However, many cancer cell types are known to have resistance to ROS-induced chemotherapy drugs, partly via increasing the expression of some of key antioxidant genes. For instance, sorafenib resistance (both primary and acquired), in which upregulation of NRF2-related signaling pathway in cancer cells plays a core role, is considered to be the main cause of poor prognosis in HCC patients. Currently, some novel treatment strategies to overcome drug resistance has been developed [144]. For instance, the combination therapy of metformin and sorafenib on HCC cells can induce ferroptosis through the p62-Keap1-NRF2 antioxidant signaling pathway. By preventing the translocation of NRF2, metformin reduces HO-1 expression, making HCC cells more sensitive to sorafenib and enhancing its anti-tumor effect [122]. Artesunate can also be used to sensitize sorafenib-induced ferroptosis by promoting lysosomal function [145].
Some nutraceuticals composed of active chemicals derived from natural sources are also capable of combating HCC. For example, APG (Apigenin, 4′,5,7-trihydroxyflavone), a natural bioflavonoid extensively existing in numerous fruits and vegetables, can achieve anti-tumor effect by altering the miR-101/NRF2-related pathway [123]. As a post-transcriptional regulator, miRNAs (microRNAs), a type of noncoding RNA with the length of 18–25 nucleotides, target the 3′-UTRs of mRNA, inhibiting its translation or causing its degradation [146]. As for the pharmacological mechanisms of APG, it is able to upregulate the expression of miR-101 [123], which implies that APG can attenuate the expression of NRF2 at the protein and mRNA levels and reduce NRF2 target genes expression, thus inducing ferroptosis of tumor cells.
-
(2).
Breast cancer
About 1.7 million people worldwide are diagnosed with breast cancer every year, and about 500,000 people die of breast cancer [147]. (1S,3R)-RSL3 can inhibit the peroxidase activity of GPX4 in BC (breast cancer) cells, causing the accumulation of peroxides and then ferroptosis [124]. It is worth mentioning that drug-resistant breast cancer cells often show stronger dependence on GPX4 and are more sensitive to GPX4 inhibitors [148]. Therefore, GPX4 can be a potential target to overcome drug resistance in breast cancer.
Fascin is able to enhance the vulnerability of breast cancer to erastin-induced ferroptosis by degrading xCT through ubiquitin-mediated proteasome degradation pathway [125]. In addition, siramesine, a lysosomotropic agent, and lapatinib, a potent double tyrosine kinase inhibitor of EGFR (epidermal growth factor receptor, ErbB-1) and ErbB-2 [114], can co-operatively induce ferroptosis-mediated cell death in BC cells [149,150]. Concerning the underlying mechanism, Ma et al. [150] demonstrated that the combination of siramesine and lapatinib attenuates the expression of ferroportin and ferritin and promotes the expression of transferrin, causing an increase in intracellular iron ion levels. Excess iron then participates in the Fenton reaction, raising the generation of ROS and leading to ferroptosis in MDA MB 231 and SKBR3 cells.
Moreover, LA (Levistilide A), an active compound extracted from Chuanxiong Rhizoma, is extensively utilized in cancer treatment in traditional oriental medicine. Previous studies have suggested that it is capable of promoting apoptosis in colon cancer cells through the ER (endoplasmic reticulum) stress pathway induced by ROS accumulation, exerting an anti-tumor effect [151]. In 2022, a study explored the underlying mechanism of LA-induced ferroptosis in vitro and eventually confirmed that LA treatment activated NRF2/HO-1 signaling pathway (upregulation of the expression of NRF2 and its downstream molecule HO-1), which enhanced ROS-induced ferroptosis in BC cells due to the dual effects of HO-1. Furthermore, LA also eliminate BC cells by significantly decreasing cell viability in a dose-dependent manner and disrupting the structure and function of mitochondria [131].
-
(3).
Lung cancer
Lung cancer is responsible for massive cancer-related deaths worldwide. In 2018, the global incidence of lung cancer took the lead among all kinds of cancers [152]. NSCLC (non-small-cell lung cancer) is the most common type of lung cancer, representing over 80% of all lung cancers. DDP (Cisplatin) is regarded as the first-in-class drug for advanced and non-targetable NSCLC. Studies have indicated that DDP exerts the ability of inducing both ferroptosis and apoptosis in the NSCLC cell lines A549 and HCT116 cells [110]. It is confirmed that the major part of intracellular DDP can bind to GSH, forming the Pt-GS complex [153]. Therefore, similar to erastin, via GSH depletion and the inactivation of GPXs, DDP induces ferroptosis of cancer cells. However, it is essential to mention that anti-tumor effects of the combined application of erastin and DDP surpasses that of single use of DDP [110].
However, drug resistance to DDP is a major barrier to lung cancer treatment as well. A recent study revealed that the combination of isoorientin (also known as homoorientin) and DDP treatment resulted in an apparent decrease in the viability of drug-resistant cells through in vitro and in vivo experiments. In terms of mechanism, isoorientin acts as a modulator that controls the SIRT6/NRF2/GPX4 signaling pathway (downregulation of the expression of SIRT6 protein, NRF2, and GPX4), thereby regulating ferroptosis and reversing drug resistance in lung cancer cells [126].
A new drug, ZVI-NP (zero-valent-iron nanoparticle), which has been widely developed to treat contaminated groundwater or wastewater [154], is now gradually coming into sight for its anti-tumor efficacy. Previously, ZVI-NP has been found to be preferentially converted to ferric ions in lysosomes of cancer cells rather than in normal cells, since cancer cells are more acidic in intra-organelle compartments. Sudden release of iron ions further induced a ROS surge in cancer cells, and led to ferroptosis in cancer cells [155]. In 2021, when exploring the potential role of ZVI-NP in regulating the tumor microenvironment in lung cancer models, a study identified a novel molecular mechanism in inducing ferroptosis. Mechanistically, ZVI-NP enhanced phosphate-dependent ubiquitination and degradation of NRF2, thereby triggering ferroptosis along with excessive oxidative stress and lipid peroxidation. Moreover, ZVI-NP impaired the self-renewal capacity of cancer cells and inhibited angiogenesis in endothelial cells [127], all of which profoundly opened up the potential of new advanced cancer therapies that reduced side effects and enhanced efficacy.
-
(4).
Pancreatic cancer
Pancreatic cancer ranks seventh in cancer-related mortality according to a survey conducted by the American Cancer Society in 2021 [156]. Moreover, a study has indicated that pancreatic carcinoma will represent the second leading cause of cancer-related deaths in the United States by 2030 [157]. Wogonin, as a main element composing Scutellaria baicalensis [158], is found to exhibit strong anti-inflammatory activity and potential for treating tumors in vitro and in vivo. A recent study has shown that wogonin decreases the GSH content in cells by suppressing the NRF2-mediated GPX4 pathway, leading to ROS accumulation and the enhancement of lipid peroxidation in pancreatic cancer cells [128], which indicates that wogonin could be potentially used for the treatment of pancreatic carcinoma.
-
(5).
Other cancers
AS (Artesunate), a water-soluble derivative of artemisinin, induces ferroptosis in head and neck cancer cells by depleting GSH and causing ROS accumulation. Furthermore, silence of NRF2 by RNA interference or inhibition of NRF2 by trigonelline can enhance the killing effect of AS on drug-resistant head and neck cancer cells in vivo and in vitro [37]. Sulfasalazine is an azo-bridged agent with anti-inflammatory activity, originally synthesized from the antibiotic sulfapyridine in 1940. It was confirmed to be a potent inhibitor of xCT in 2001 [129]. Through disturbing cystine absorption, sulfasalazine mitigates the biosynthesis of GSH, resulting in ferroptosis [4] of some types of cancer cells in vivo and in vitro [130], such as lymphoma [129]. Sulfasalazine is supposed to be non-toxic so that it can be utilized in combination with other drugs to fight against drug resistance and side effects [159]. Okazaki et al. [160] found that the combination of dyclonine and sulfasalazine restrained the growth of head and neck squamous cell carcinoma or gastric tumors with high expression of ALDH3A1, which contributes to resistance to sulfasalazine monotherapy. Tagitinin C, a sesquiterpene lactone isolated from Tithonia diversifolia, is widely found in the Asteraceae [161], with ample pharmacological effects, including anti-tumor, anti-virus, anti-fibrosis, anti-parasite, and cardio-protective activity [162,163,164]. Mechanistically, it induced ER stress and oxidative stress, which activated the nuclear translocation of NRF2, resulting in oxidative cell microenvironment, ferroptosis, and suppressed growth of colorectal cancer cells. As a downstream effector of NRF2, the expression of HO-1 increased significantly, which led to an increase in the LIP and promoted lipid peroxidation. Furthermore, tagitinine C exhibited synergistic anti-tumor effects together with the erastin [132].
In general, it is reasonable to believe that more and more treatment strategies with higher efficacy and prognosis will be found in the future. The application of drugs that target NRF2 signaling pathway and induce ferroptosis to kill tumors is both novel and promising.
4.2. Targeting of NRF2 Signaling Pathway and Inhibition of Ferroptosis in Disease Therapy
4.2.1. Diseases Associated with Ferroptosis
-
(1).
Neurodegenerative diseases
Ferroptosis is presented in Alzheimer’s disease, Parkinson’s disease, and many other neurodegenerative diseases. Iron is the most abundant transition metal in the brain, participating in numerous metabolic reactions. Disorder of iron homeostasis in the brain can lead to some neurodegenerative diseases, such as Alzheimer’s disease and Parkinson’s disease [165,166,167]. Iron gradually accumulates in the brain with age, which changes the cerebral iron metabolism. In animal models of neurodegenerative diseases as well as aging populations, varying degrees of elevation of iron content in the brain have been observed [168,169]. Studies also show that chronic exposure to iron for mice causes dysfunction of membrane-transport protein, imbalance of intracellular iron homeostasis, and significant elevation of ROS and MDA (malondialdehyde), which ultimately lead to the dysfunction of nervous system [170]. Numerous studies reveal that disorder of iron metabolism has an influence on the normal folding of Aβ protein (amyloid β-protein) and phosphorylation of tau protein, resulting in oxidative stress and metal toxicity, which can cause DNA, lipid, and protein damage and ultimately lead to Alzheimer’s disease [171,172,173]. Studies also indicate that cognitive function of Alzheimer’s patients can be stabilized when applying antioxidants and iron chelators like α-lipoic acid, which blocks tau-induced iron overload, lipid peroxidation, and ferroptosis-related inflammation [174]. Parkinson disease is characterized by mitochondrial dysfunction, iron accumulation, copper depletion, and GSH exhaustion in the brain, some of which are also the most important features of ferroptosis [175,176,177,178,179]. Iron chelators (ferroptosis inhibitor) like deferiprone show therapeutic effect in Parkinson’s patients in a randomized controlled trial [180].
-
(2).
Cardiovascular diseases
Ferroptosis plays an influential role in cardiovascular diseases. Recent studies have found a significant decrease in GPX4 expression in the early and middle stages of myocardial infarction [181]. Human umbilical cord blood-derived mesenchymal stem cell exosomes may inhibit ferroptosis by targeting miR-23a-3p, thereby achieving a protective effect in alleviating acute myocardial infarction [182]. Ferroptosis also plays an important role in hypertension. Pulmonary hypertension can cause a decrease in GSH levels and an increase in iron, ROS, and lipid peroxides [183]. The cardiotoxicity of anthracyclines (e.g., doxorubicin) is also associated with ferroptosis. Studies have shown that doxorubicin can lead to heme degradation, causing mitochondrial dysfunction and a significant increase in the content of iron, ROS, and other markers of ferroptosis [35]. In DOX (doxorubicin)-induced cardiomyopathy, the administration of ferroptosis inhibitor Fer-1 (ferrostatin-1) can significantly reduce the mortality rate of mice, while the mortality rate of mice administered with inhibitors of apoptosis, necrosis, and autophagy is not significantly reduced [35]. Ferroptosis may also play a significant role in sepsis. In animal models of sepsis with cecum ligation and puncture, the levels of GSH and GPX4 decreased accompanied by elevated levels of iron and lipid peroxides [184]. Many other types of cardiovascular diseases, such as arrhythmias and sickle cell anemia, are also considered to be associated with ferroptosis [185,186].
-
(3).
Organic injuries
Ferroptosis has been investigated in many organic injuries as well. For example, in mice with sepsis, multiple organic injuries caused by ferroptosis can be detected. Ferroptosis has also been proved to play an important role in LPS-induced acute lung injury, in which Fer-1 also displays effective therapeutic effect [187]. In various acute renal injuries, like rhabdomyolysis-induced acute kidney injury and folic acid-induced acute kidney injury, iron accumulation and ROS have been observed and can be suppressed by ferroptosis inhibitors [188,189,190]. Fe2+ produced by myoglobin metabolism directly induces lipid peroxidation in proximal tubular epithelial cells in rhabdomyolysis, which may be an important mechanism of rhabdomyolysis inducing acute kidney injury [189]. And the application of iron chelator deferoxamine is able to alleviate rhabdomyolysis-induced kidney injury in mice [191].
4.2.2. Drugs and Clinical Experiments
There have been many studies focusing on the role of NRF2 and showing that regulation of NRF2 and related signaling pathway can alleviate neurodegeneration, cardiovascular diseases, and many organic injuries.
-
(1).
Neurodegenerative diseases
NRF2 has promising prospects as a novel target for the treatment of neurodegenerative diseases, since Alzheimer’s disease is closely related to oxidative stress, mitochondrial dysfunction, and ferroptosis. The activation of NRF2 can upregulate the expression of xCT, enhance glutamate secretion, and restore GPX4 activity, thereby inhibiting ferroptosis. Therefore, drugs activating NRF2 could become a new approach to treat Alzheimer’s disease. DMF (Tecfidera®) can be regarded as the most successful case reported in activating NRF2 [192,193]. The drug is originally used to treat psoriasis patients, but new research shows that after entering the body, it transformed so as to covalently modify key cysteine residues on KEAP1, thus blocking NRF2 ubiquitination and promoting NRF2 stabilization as well as subsequent activation of NRF2 target genes [133]. In addition, many drugs that can be used to treat Alzheimer’s disease by activating NRF2, such as resveratrol, pyridoxine, and NBP (n-butylphthalide), have also entered clinical trials.
-
(2).
Cardiovascular diseases
Targeting NRF2 has broad prospects in the treatment of cardiovascular diseases. SIRT1 (Sirtuin1) is a NAD-dependent deacetylase which plays an important role in a variety of cardiovascular diseases caused by diabetes. It can increase the localization of NRF2 in the nucleus, thereby alleviating myocardial ischemia-reperfusion injury in the diabetic Sprague Dawley rat model [134]. The effect of SIRT1 may be achieved by deacetylation of NRF2 and reduction of NRF2 ubiquitination [194]. At the same time, miRNAs can indirectly regulate NRF2 expression by modulating the upstream protein NRF2. MiR-24-3p that has been shown to modulate the Keap1/NRF2 pathway during myocardial ischemia-reperfusion [135]. In highly glucose-stimulated rat and mouse cardiomyocyte models, inhibition of miR-144, miR-155, and miR-503 activates NRF2 to attenuate cellular oxidative stress [195,196,197]. MiR-24 has been shown to activate the NRF2/HO-1 signaling pathway, playing a critical role in combating oxidative stress in VSMCs (vascular smooth muscle cells) stimulated by high glucose [198].
-
(3).
Organic injuries
In brain injury related to transient global cerebral ischemia in rats, study shows that preischemia dexmedetomidine administration has neuroprotective effect by enhancing NRF2/HO-1 expression and reducing caspase-3 activity [136]. In ischemia brain injury, BCP (β-Caryophyllene) has protective effects, whose mechanism is related to the activation of the NRF2/HO-1 pathway [137]. Moreover, study shows that XJZ (Xiaojianzhong decoction), which consists of six Chinese herbal medicines and extracts, can attenuate ferroptosis mediated by oxidative stress in the gastric mucosal injury by upregulating the p62/Keap1/NRF2 pathway [138]. In sepsis-induced lung injury, AS (artesunate) upregulates the expression of antiferroptosis systems like NRF2 and HO-1, thus attenuating neutrophil infiltration and pathological damage in lung tissue [139]. Studies in the type II diabetic nephropathy rat model show that experimental diabetic nephric injury can be alleviated by targeting the Keap1/NRF2 pathway using MiR-200a [140].
Although drugs targeting NRF2 show therapeutic functions, and have a promising future, studies on these drugs are still limited to the experimental stage, and further clinical trials are needed for application. Multiple aspects, including safety, efficacy, and economy, should be taken into consideration before clinical application.
5. Conclusions and Future Perspectives
With a further understanding of the mechanism of ferroptosis, the criticality of NRF2 is demonstrated more obviously, whose rich value embodies in both antioxidant-dependent and antioxidant-independent functions. As a key regulator of the cellular antioxidant response, NRF2 is widely involved in iron, lipid, amino acid, glucose metabolisms, etc. Therefore, through pharmacological modulation of NRF2 and its downstream effectors, prevention and treatment of pathologies, such as numerous types of cancers, neurodegenerative diseases, cardiovascular diseases, and so on, can be achieved. Despite several unclear issues, such as the specific connection between lipid peroxidation and ferroptosis, there is no denying the fact that therapies targeting NRF2 signaling pathway exert extraordinary efficacy. This kind of approach can be used to treat various ferroptosis-related diseases and may have a brilliant therapeutic future.
Author Contributions
R.Y., B.L., W.J., L.T. and R.C. figured out the idea of writing this review. R.Y., B.L., W.J. and L.T. summarized the published results and drafted the manuscript. S.H. and R.C. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The review was funded by the Innovative Research Team of High-level Local Universities in Shanghai and supported by the National Natural Science Foundation of China (81872230 and 82173352 to R.C.).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Dolma S., Lessnick S.L., Hahn W.C., Stockwell B.R. Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell. 2003;3:285–296. doi: 10.1016/S1535-6108(03)00050-3. [DOI] [PubMed] [Google Scholar]
- 2.Yagoda N., von Rechenberg M., Zaganjor E., Bauer A.J., Yang W.S., Fridman D.J., Wolpaw A.J., Smukste I., Peltier J.M., Boniface J.J., et al. RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature. 2007;447:864–868. doi: 10.1038/nature05859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang W.S., Stockwell B.R. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem. Biol. 2008;15:234–245. doi: 10.1016/j.chembiol.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dixon S.J., Lemberg K.M., Lamprecht M.R., Skouta R., Zaitsev E.M., Gleason C.E., Patel D.N., Bauer A.J., Cantley A.M., Yang W.S., et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedmann Angeli J.P., Schneider M., Proneth B., Tyurina Y.Y., Tyurin V.A., Hammond V.J., Herbach N., Aichler M., Walch A., Eggenhofer E., et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 2014;16:1180–1191. doi: 10.1038/ncb3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doll S., Proneth B., Tyurina Y.Y., Panzilius E., Kobayashi S., Ingold I., Irmler M., Beckers J., Aichler M., Walch A., et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Chem. Biol. 2017;13:91–98. doi: 10.1038/nchembio.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maiorino M., Conrad M., Ursini F. GPx4, Lipid Peroxidation, and Cell Death: Discoveries, Rediscoveries, and Open Issues. Antioxid. Redox Signal. 2018;29:61–74. doi: 10.1089/ars.2017.7115. [DOI] [PubMed] [Google Scholar]
- 8.Ingold I., Berndt C., Schmitt S., Doll S., Poschmann G., Buday K., Roveri A., Peng X., Porto Freitas F., Seibt T., et al. Selenium Utilization by GPX4 Is Required to Prevent Hydroperoxide-Induced Ferroptosis. Cell. 2018;172:409–422.e21. doi: 10.1016/j.cell.2017.11.048. [DOI] [PubMed] [Google Scholar]
- 9.Moi P., Chan K., Asunis I., Cao A., Kan Y.W. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc. Natl. Acad. Sci. USA. 1994;91:9926–9930. doi: 10.1073/pnas.91.21.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Itoh K., Chiba T., Takahashi S., Ishii T., Igarashi K., Katoh Y., Oyake T., Hayashi N., Satoh K., Hatayama I., et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 11.Sasaki H., Sato H., Kuriyama-Matsumura K., Sato K., Maebara K., Wang H., Tamba M., Itoh K., Yamamoto M., Bannai S. Electrophile response element-mediated induction of the cystine/glutamate exchange transporter gene expression. J. Biol. Chem. 2002;277:44765–44771. doi: 10.1074/jbc.M208704200. [DOI] [PubMed] [Google Scholar]
- 12.Lee J.M., Calkins M.J., Chan K., Kan Y.W., Johnson J.A. Identification of the NF-E2-related factor-2-dependent genes conferring protection against oxidative stress in primary cortical astrocytes using oligonucleotide microarray analysis. J. Biol. Chem. 2003;278:12029–12038. doi: 10.1074/jbc.M211558200. [DOI] [PubMed] [Google Scholar]
- 13.Wu K.C., Cui J.Y., Klaassen C.D. Beneficial role of Nrf2 in regulating NADPH generation and consumption. Toxicol. Sci. 2011;123:590–600. doi: 10.1093/toxsci/kfr183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kerins M.J., Ooi A. The Roles of NRF2 in Modulating Cellular Iron Homeostasis. Antioxid. Redox Signal. 2018;29:1756–1773. doi: 10.1089/ars.2017.7176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Porter N.A., Caldwell S.E., Mills K.A. Mechanisms of free radical oxidation of unsaturated lipids. Lipids. 1995;30:277–290. doi: 10.1007/BF02536034. [DOI] [PubMed] [Google Scholar]
- 16.Zou Y., Li H., Graham E.T., Deik A.A., Eaton J.K., Wang W., Sandoval-Gomez G., Clish C.B., Doench J.G., Schreiber S.L. Cytochrome P450 oxidoreductase contributes to phospholipid peroxidation in ferroptosis. Nat. Chem. Biol. 2020;16:302–309. doi: 10.1038/s41589-020-0472-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuhn H., Banthiya S., van Leyen K. Mammalian lipoxygenases and their biological relevance. Biochim. Biophys. Acta. 2015;1851:308–330. doi: 10.1016/j.bbalip.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kagan V.E., Mao G., Qu F., Angeli J.P., Doll S., Croix C.S., Dar H.H., Liu B., Tyurin V.A., Ritov V.B., et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat. Chem. Biol. 2017;13:81–90. doi: 10.1038/nchembio.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doll S., Freitas F.P., Shah R., Aldrovandi M., da Silva M.C., Ingold I., Goya Grocin A., Xavier da Silva T.N., Panzilius E., Scheel C.H., et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature. 2019;575:693–698. doi: 10.1038/s41586-019-1707-0. [DOI] [PubMed] [Google Scholar]
- 20.Lee H., Zandkarimi F., Zhang Y., Meena J.K., Kim J., Zhuang L., Tyagi S., Ma L., Westbrook T.F., Steinberg G.R., et al. Energy-stress-mediated AMPK activation inhibits ferroptosis. Nat. Cell Biol. 2020;22:225–234. doi: 10.1038/s41556-020-0461-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shimada K., Skouta R., Kaplan A., Yang W.S., Hayano M., Dixon S.J., Brown L.M., Valenzuela C.A., Wolpaw A.J., Stockwell B.R. Global survey of cell death mechanisms reveals metabolic regulation of ferroptosis. Nat. Chem. Biol. 2016;12:497–503. doi: 10.1038/nchembio.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Küch E.M., Vellaramkalayil R., Zhang I., Lehnen D., Brügger B., Sreemmel W., Ehehalt R., Poppelreuther M., Füllekrug J. Differentially localized acyl-CoA synthetase 4 isoenzymes mediate the metabolic channeling of fatty acids towards phosphatidylinositol. Biochim. Biophys. Acta. 2014;1841:227–239. doi: 10.1016/j.bbalip.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 23.Shindou H., Shimizu T. Acyl-CoA:lysophospholipid acyltransferases. J. Biol. Chem. 2009;284:1–5. doi: 10.1074/jbc.R800046200. [DOI] [PubMed] [Google Scholar]
- 24.Wu J., Minikes A.M., Gao M., Bian H., Li Y., Stockwell B.R., Chen Z.N., Jiang X. Intercellular interaction dictates cancer cell ferroptosis via NF2-YAP signalling. Nature. 2019;572:402–406. doi: 10.1038/s41586-019-1426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frazer D.M., Anderson G.J. The regulation of iron transport. Biofactors. 2014;40:206–214. doi: 10.1002/biof.1148. [DOI] [PubMed] [Google Scholar]
- 26.Ohgami R.S., Campagna D.R., Greer E.L., Antiochos B., McDonald A., Chen J., Sharp J.J., Fujiwara Y., Barker J.E., Fleming M.D. Identification of a ferrireductase required for efficient transferrin-dependent iron uptake in erythroid cells. Nat. Genet. 2005;37:1264–1269. doi: 10.1038/ng1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fleming M.D., Romano M.A., Su M.A., Garrick L.M., Garrick M.D., Andrews N.C. Nramp2 is mutated in the anemic Belgrade (b) rat: Evidence of a role for Nramp2 in endosomal iron transport. Proc. Natl. Acad. Sci. USA. 1998;95:1148–1153. doi: 10.1073/pnas.95.3.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel S.J., Frey A.G., Palenchar D.J., Achar S., Bullough K.Z., Vashisht A., Wohlschlegel J.A., Philpott C.C. A PCBP1-BolA2 chaperone complex delivers iron for cytosolic [2Fe-2S] cluster assembly. Nat. Chem. Biol. 2019;15:872–881. doi: 10.1038/s41589-019-0330-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson G.J., Vulpe C.D. Mammalian iron transport. Cell Mol. Life Sci. 2009;66:3241–3261. doi: 10.1007/s00018-009-0051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bogdan A.R., Miyazawa M., Hashimoto K., Tsuji Y. Regulators of Iron Homeostasis: New Players in Metabolism, Cell Death, and Disease. Trends Biochem. Sci. 2016;41:274–286. doi: 10.1016/j.tibs.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Campbell M.R., Karaca M., Adamski K.N., Chorley B.N., Wang X., Bell D.A. Novel hematopoietic target genes in the NRF2-mediated transcriptional pathway. Oxid. Med. Cell Longev. 2013;2013:120305. doi: 10.1155/2013/120305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Agyeman A.S., Chaerkady R., Shaw P.G., Davidson N.E., Visvanathan K., Pandey A., Kensler T.W. Transcriptomic and proteomic profiling of KEAP1 disrupted and sulforaphane-treated human breast epithelial cells reveals common expression profiles. Breast Cancer Res. Treat. 2012;132:175–187. doi: 10.1007/s10549-011-1536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirotsu Y., Katsuoka F., Funayama R., Nagashima T., Nishida Y., Nakayama K., Engel J.D., Yamamoto M. Nrf2-MafG heterodimers contribute globally to antioxidant and metabolic networks. Nucleic Acids Res. 2012;40:10228–10239. doi: 10.1093/nar/gks827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hassannia B., Wiernicki B., Ingold I., Qu F., Van Herck S., Tyurina Y.Y., Bayır H., Abhari B.A., Angeli J.P.F., Choi S.M., et al. Nano-targeted induction of dual ferroptotic mechanisms eradicates high-risk neuroblastoma. J. Clin. Investig. 2018;128:3341–3355. doi: 10.1172/JCI99032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fang X., Wang H., Han D., Xie E., Yang X., Wei J., Gu S., Gao F., Zhu N., Yin X., et al. Ferroptosis as a target for protection against cardiomyopathy. Proc. Natl. Acad. Sci. USA. 2019;116:2672–2680. doi: 10.1073/pnas.1821022116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adedoyin O., Boddu R., Traylor A., Lever J.M., Bolisetty S., George J.F., Agarwal A. Heme oxygenase-1 mitigates ferroptosis in renal proximal tubule cells. Am. J. Physiol. Renal Physiol. 2018;314:F702–F714. doi: 10.1152/ajprenal.00044.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roh J.L., Kim E.H., Jang H., Shin D. Nrf2 inhibition reverses the resistance of cisplatin-resistant head and neck cancer cells to artesunate-induced ferroptosis. Redox Biol. 2017;11:254–262. doi: 10.1016/j.redox.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun X., Ou Z., Chen R., Niu X., Chen D., Kang R., Tang D. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology. 2016;63:173–184. doi: 10.1002/hep.28251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown C.W., Amante J.J., Chhoy P., Elaimy A.L., Liu H., Zhu L.J., Baer C.E., Dixon S.J., Mercurio A.M. Prominin2 Drives Ferroptosis Resistance by Stimulating Iron Export. Dev. Cell. 2019;51:575–586.e4. doi: 10.1016/j.devcel.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anandhan A., Dodson M., Shakya A., Chen J., Liu P., Wei Y., Tan H., Wang Q., Jiang Z., Yang K., et al. NRF2 controls iron homeostasis and ferroptosis through HERC2 and VAMP8. Sci. Adv. 2023;9:eade9585. doi: 10.1126/sciadv.ade9585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anandhan A., Dodson M., Schmidlin C.J., Liu P., Zhang D.D. Breakdown of an Ironclad Defense System: The Critical Role of NRF2 in Mediating Ferroptosis. Cell Chem. Biol. 2020;27:436–447. doi: 10.1016/j.chembiol.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pajares M., Jiménez-Moreno N., García-Yagüe Á.J., Escoll M., de Ceballos M.L., Van Leuven F., Rábano A., Yamamoto M., Rojo A.I., Cuadrado A. Transcription factor NFE2L2/NRF2 is a regulator of macroautophagy genes. Autophagy. 2016;12:1902–1916. doi: 10.1080/15548627.2016.1208889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao M., Yi J., Zhu J., Minikes A.M., Monian P., Thompson C.B., Jiang X. Role of Mitochondria in Ferroptosis. Mol. Cell. 2019;73:354–363.e3. doi: 10.1016/j.molcel.2018.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tong K.I., Katoh Y., Kusunoki H., Itoh K., Tanaka T., Yamamoto M. Keap1 recruits Neh2 through binding to ETGE and DLG motifs: Characterization of the two-site molecular recognition model. Mol. Cell Biol. 2006;26:2887–2900. doi: 10.1128/MCB.26.8.2887-2900.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hayano M., Yang W.S., Corn C.K., Pagano N.C., Stockwell B.R. Loss of cysteinyl-tRNA synthetase (CARS) induces the transsulfuration pathway and inhibits ferroptosis induced by cystine deprivation. Cell Death Differ. 2016;23:270–278. doi: 10.1038/cdd.2015.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seibt T.M., Proneth B., Conrad M. Role of GPX4 in ferroptosis and its pharmacological implication. Free Radic. Biol. Med. 2019;133:144–152. doi: 10.1016/j.freeradbiomed.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 47.Hawkes H.J., Karlenius T.C., Tonissen K.F. Regulation of the human thioredoxin gene promoter and its key substrates: A study of functional and putative regulatory elements. Biochim. Biophys. Acta. 2014;1840:303–314. doi: 10.1016/j.bbagen.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 48.Malhotra D., Portales-Casamar E., Singh A., Srivastava S., Arenillas D., Happel C., Shyr C., Wakabayashi N., Kensler T.W., Wasserman W.W., et al. Global mapping of binding sites for Nrf2 identifies novel targets in cell survival response through ChIP-Seq profiling and network analysis. Nucleic Acids Res. 2010;38:5718–5734. doi: 10.1093/nar/gkq212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiang L., Hickman J.H., Wang S.J., Gu W. Dynamic roles of p53-mediated metabolic activities in ROS-induced stress responses. Cell Cycle. 2015;14:2881–2885. doi: 10.1080/15384101.2015.1068479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Friedmann Angeli J.P., Conrad M. Selenium and GPX4, a vital symbiosis. Free Radic. Biol. Med. 2018;127:153–159. doi: 10.1016/j.freeradbiomed.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 51.Melo A.M., Bandeiras T.M., Teixeira M. New insights into type II NAD(P)H:quinone oxidoreductases. Microbiol. Mol. Biol. Rev. 2004;68:603–616. doi: 10.1128/MMBR.68.4.603-616.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Warner G.J., Berry M.J., Moustafa M.E., Carlson B.A., Hatfield D.L., Faust J.R. Inhibition of selenoprotein synthesis by selenocysteine tRNA[Ser]Sec lacking isopentenyladenosine. J. Biol. Chem. 2000;275:28110–28119. doi: 10.1074/jbc.M001280200. [DOI] [PubMed] [Google Scholar]
- 53.Venkatesh D., O’Brien N.A., Zandkarimi F., Tong D.R., Stokes M.E., Dunn D.E., Kengmana E.S., Aron A.T., Klein A.M., Csuka J.M., et al. MDM2 and MDMX promote ferroptosis by PPARα-mediated lipid remodeling. Genes. Dev. 2020;34:526–543. doi: 10.1101/gad.334219.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kraft V.A.N., Bezjian C.T., Pfeiffer S., Ringelstetter L., Müller C., Zandkarimi F., Merl-Pham J., Bao X., Anastasov N., Kössl J., et al. GTP Cyclohydrolase 1/Tetrahydrobiopterin Counteract Ferroptosis through Lipid Remodeling. ACS Cent. Sci. 2020;6:41–53. doi: 10.1021/acscentsci.9b01063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paton C.M., Ntambi J.M. Biochemical and physiological function of stearoyl-CoA desaturase. Am. J. Physiol. Endocrinol. Metab. 2009;297:E28–E37. doi: 10.1152/ajpendo.90897.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Magtanong L., Ko P.J., To M., Cao J.Y., Forcina G.C., Tarangelo A., Ward C.C., Cho K., Patti G.J., Nomura D.K., et al. Exogenous Monounsaturated Fatty Acids Promote a Ferroptosis-Resistant Cell State. Cell Chem. Biol. 2019;26:420–432.e9. doi: 10.1016/j.chembiol.2018.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Katoh Y., Itoh K., Yoshida E., Miyagishi M., Fukamizu A., Yamamoto M. Two domains of Nrf2 cooperatively bind CBP, a CREB binding protein, and synergistically activate transcription. Genes Cells. 2001;6:857–868. doi: 10.1046/j.1365-2443.2001.00469.x. [DOI] [PubMed] [Google Scholar]
- 58.Katsuoka F., Motohashi H., Ishii T., Aburatani H., Engel J.D., Yamamoto M. Genetic evidence that small Maf proteins are essential for the activation of antioxidant response element-dependent genes. Mol. Cell. Biol. 2005;25:8044–8051. doi: 10.1128/MCB.25.18.8044-8051.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kobayashi M., Itoh K., Suzuki T., Osanai H., Nishikawa K., Katoh Y., Takagi Y., Yamamoto M. Identification of the interactive interface and phylogenic conservation of the Nrf2-Keap1 system. Genes Cells. 2002;7:807–820. doi: 10.1046/j.1365-2443.2002.00561.x. [DOI] [PubMed] [Google Scholar]
- 60.McMahon M., Itoh K., Yamamoto M., Hayes J.D. Keap1-dependent proteasomal degradation of transcription factor Nrf2 contributes to the negative regulation of antioxidant response element-driven gene expression. J. Biol. Chem. 2003;278:21592–21600. doi: 10.1074/jbc.M300931200. [DOI] [PubMed] [Google Scholar]
- 61.Nioi P., Nguyen T., Sherratt P.J., Pickett C.B. The carboxy-terminal Neh3 domain of Nrf2 is required for transcriptional activation. Mol. Cell. Biol. 2005;25:10895–10906. doi: 10.1128/MCB.25.24.10895-10906.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moore S., Berger N.D., Luijsterburg M.S., Piett C.G., Stanley F.K.T., Schräder C.U., Fang S., Chan J.A., Schriemer D.C., Nagel Z.D., et al. The CHD6 chromatin remodeler is an oxidative DNA damage response factor. Nat. Commun. 2019;10:241. doi: 10.1038/s41467-018-08111-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Salazar M., Rojo A.I., Velasco D., de Sagarra R.M., Cuadrado A. Glycogen synthase kinase-3beta inhibits the xenobiotic and antioxidant cell response by direct phosphorylation and nuclear exclusion of the transcription factor Nrf2. J. Biol. Chem. 2006;281:14841–14851. doi: 10.1074/jbc.M513737200. [DOI] [PubMed] [Google Scholar]
- 64.Nam L.B., Keum Y.S. Binding partners of NRF2: Functions and regulatory mechanisms. Arch. Biochem. Biophys. 2019;678:108184. doi: 10.1016/j.abb.2019.108184. [DOI] [PubMed] [Google Scholar]
- 65.Cuadrado A. Structural and functional characterization of Nrf2 degradation by glycogen synthase kinase 3/beta-TrCP. Free Radic. Biol. Med. 2015;88:147–157. doi: 10.1016/j.freeradbiomed.2015.04.029. [DOI] [PubMed] [Google Scholar]
- 66.Wang X.J., Hayes J.D., Henderson C.J., Wolf C.R. Identification of retinoic acid as an inhibitor of transcription factor Nrf2 through activation of retinoic acid receptor alpha. Proc. Natl. Acad. Sci. USA. 2007;104:19589–19594. doi: 10.1073/pnas.0709483104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang H., Liu K., Geng M., Gao P., Wu X., Hai Y., Li Y., Li Y., Luo L., Hayes J.D., et al. RXRα inhibits the NRF2-ARE signaling pathway through a direct interaction with the Neh7 domain of NRF2. Cancer Res. 2013;73:3097–3108. doi: 10.1158/0008-5472.CAN-12-3386. [DOI] [PubMed] [Google Scholar]
- 68.Levonen A.L., Landar A., Ramachandran A., Ceaser E.K., Dickinson D.A., Zanoni G., Morrow J.D., Darley-Usmar V.M. Cellular mechanisms of redox cell signalling: Role of cysteine modification in controlling antioxidant defences in response to electrophilic lipid oxidation products. Biochem. J. 2004;378:373–382. doi: 10.1042/bj20031049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Komatsu M., Kurokawa H., Waguri S., Taguchi K., Kobayashi A., Ichimura Y., Sou Y.S., Ueno I., Sakamoto A., Tong K.I., et al. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat. Cell Biol. 2010;12:213–223. doi: 10.1038/ncb2021. [DOI] [PubMed] [Google Scholar]
- 70.Rada P., Rojo A.I., Chowdhry S., McMahon M., Hayes J.D., Cuadrado A. SCF/{beta}-TrCP promotes glycogen synthase kinase 3-dependent degradation of the Nrf2 transcription factor in a Keap1-independent manner. Mol. Cell Biol. 2011;31:1121–1133. doi: 10.1128/MCB.01204-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rada P., Rojo A.I., Evrard-Todeschi N., Innamorato N.G., Cotte A., Jaworski T., Tobón-Velasco J.C., Devijver H., García-Mayoral M.F., Van Leuven F., et al. Structural and functional characterization of Nrf2 degradation by the glycogen synthase kinase 3/β-TrCP axis. Mol. Cell Biol. 2012;32:3486–3499. doi: 10.1128/MCB.00180-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Martin D., Rojo A.I., Salinas M., Diaz R., Gallardo G., Alam J., De Galarreta C.M., Cuadrado A. Regulation of heme oxygenase-1 expression through the phosphatidylinositol 3-kinase/Akt pathway and the Nrf2 transcription factor in response to the antioxidant phytochemical carnosol. J. Biol. Chem. 2004;279:8919–8929. doi: 10.1074/jbc.M309660200. [DOI] [PubMed] [Google Scholar]
- 73.Wu T., Zhao F., Gao B., Tan C., Yagishita N., Nakajima T., Wong P.K., Chapman E., Fang D., Zhang D.D. Hrd1 suppresses Nrf2-mediated cellular protection during liver cirrhosis. Genes. Dev. 2014;28:708–722. doi: 10.1101/gad.238246.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Miao W., Hu L., Scrivens P.J., Batist G. Transcriptional regulation of NF-E2 p45-related factor (NRF2) expression by the aryl hydrocarbon receptor-xenobiotic response element signaling pathway: Direct cross-talk between phase I and II drug-metabolizing enzymes. J. Biol. Chem. 2005;280:20340–20348. doi: 10.1074/jbc.M412081200. [DOI] [PubMed] [Google Scholar]
- 75.Rushworth S.A., Zaitseva L., Murray M.Y., Shah N.M., Bowles K.M., MacEwan D.J. The high Nrf2 expression in human acute myeloid leukemia is driven by NF-κB and underlies its chemo-resistance. Blood. 2012;120:5188–5198. doi: 10.1182/blood-2012-04-422121. [DOI] [PubMed] [Google Scholar]
- 76.Kwak M.K., Itoh K., Yamamoto M., Kensler T.W. Enhanced expression of the transcription factor Nrf2 by cancer chemopreventive agents: Role of antioxidant response element-like sequences in the nrf2 promoter. Mol. Cell Biol. 2002;22:2883–2892. doi: 10.1128/MCB.22.9.2883-2892.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sangokoya C., Telen M.J., Chi J.T. microRNA miR-144 modulates oxidative stress tolerance and associates with anemia severity in sickle cell disease. Blood. 2010;116:4338–4348. doi: 10.1182/blood-2009-04-214817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Goldstein L.D., Lee J., Gnad F., Klijn C., Schaub A., Reeder J., Daemen A., Bakalarski C.E., Holcomb T., Shames D.S., et al. Recurrent Loss of NFE2L2 Exon 2 Is a Mechanism for Nrf2 Pathway Activation in Human Cancers. Cell Rep. 2016;16:2605–2617. doi: 10.1016/j.celrep.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 79.Ishii T., Itoh K., Takahashi S., Sato H., Yanagawa T., Katoh Y., Bannai S., Yamamoto M. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J. Biol. Chem. 2000;275:16023–16029. doi: 10.1074/jbc.275.21.16023. [DOI] [PubMed] [Google Scholar]
- 80.Yang H., Magilnick N., Lee C., Kalmaz D., Ou X., Chan J.Y., Lu S.C. Nrf1 and Nrf2 regulate rat glutamate-cysteine ligase catalytic subunit transcription indirectly via NF-kappaB and AP-1. Mol. Cell Biol. 2005;25:5933–5946. doi: 10.1128/MCB.25.14.5933-5946.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chan J.Y., Kwong M. Impaired expression of glutathione synthetic enzyme genes in mice with targeted deletion of the Nrf2 basic-leucine zipper protein. Biochim. Biophys. Acta. 2000;1517:19–26. doi: 10.1016/S0167-4781(00)00238-4. [DOI] [PubMed] [Google Scholar]
- 82.Singh A., Rangasamy T., Thimmulappa R.K., Lee H., Osburn W.O., Brigelius-Flohé R., Kensler T.W., Yamamoto M., Biswal S. Glutathione peroxidase 2, the major cigarette smoke-inducible isoform of GPX in lungs, is regulated by Nrf2. Am. J. Respir. Cell Mol. Biol. 2006;35:639–650. doi: 10.1165/rcmb.2005-0325OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jobbagy S., Vitturi D.A., Salvatore S.R., Turell L., Pires M.F., Kansanen E., Batthyany C., Lancaster J.R., Jr., Freeman B.A., Schopfer F.J. Electrophiles modulate glutathione reductase activity via alkylation and upregulation of glutathione biosynthesis. Redox Biol. 2019;21:101050. doi: 10.1016/j.redox.2018.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wu S., Lu H., Bai Y. Nrf2 in cancers: A double-edged sword. Cancer Med. 2019;8:2252–2267. doi: 10.1002/cam4.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Amaral J.H., Rizzi E.S., Alves-Lopes R., Pinheiro L.C., Tostes R.C., Tanus-Santos J.E. Antioxidant and antihypertensive responses to oral nitrite involves activation of the Nrf2 pathway. Free Radic. Biol. Med. 2019;141:261–268. doi: 10.1016/j.freeradbiomed.2019.06.028. [DOI] [PubMed] [Google Scholar]
- 86.Bersuker K., Hendricks J.M., Li Z., Magtanong L., Ford B., Tang P.H., Roberts M.A., Tong B., Maimone T.J., Zoncu R., et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019;575:688–692. doi: 10.1038/s41586-019-1705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Karlenius T.C., Tonissen K.F. Thioredoxin and Cancer: A Role for Thioredoxin in all States of Tumor Oxygenation. Cancers. 2010;2:209–232. doi: 10.3390/cancers2020209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Balsera M., Buchanan B.B. Evolution of the thioredoxin system as a step enabling adaptation to oxidative stress. Free Radic. Biol. Med. 2019;140:28–35. doi: 10.1016/j.freeradbiomed.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 89.Cuadrado A., Rojo A.I., Wells G., Hayes J.D., Cousin S.P., Rumsey W.L., Attucks O.C., Franklin S., Levonen A.L., Kensler T.W., et al. Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat. Rev. Drug Discov. 2019;18:295–317. doi: 10.1038/s41573-018-0008-x. [DOI] [PubMed] [Google Scholar]
- 90.Tonelli C., Chio I.I.C., Tuveson D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal. 2018;29:1727–1745. doi: 10.1089/ars.2017.7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Saha S., Buttari B., Panieri E., Profumo E., Saso L. An Overview of Nrf2 Signaling Pathway and Its Role in Inflammation. Molecules. 2020;25:5474. doi: 10.3390/molecules25225474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Alam J., Stewart D., Touchard C., Boinapally S., Choi A.M., Cook J.L. Nrf2, a Cap’n’Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J. Biol. Chem. 1999;274:26071–26078. doi: 10.1074/jbc.274.37.26071. [DOI] [PubMed] [Google Scholar]
- 93.Chorley B.N., Campbell M.R., Wang X., Karaca M., Sambandan D., Bangura F., Xue P., Pi J., Kleeberger S.R., Bell D.A. Identification of novel NRF2-regulated genes by ChIP-Seq: Influence on retinoid X receptor alpha. Nucleic Acids Res. 2012;40:7416–7429. doi: 10.1093/nar/gks409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hübner R.H., Schwartz J.D., De Bishnu P., Ferris B., Omberg L., Mezey J.G., Hackett N.R., Crystal R.G. Coordinate control of expression of Nrf2-modulated genes in the human small airway epithelium is highly responsive to cigarette smoking. Mol. Med. 2009;15:203–219. doi: 10.2119/molmed.2008.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mitsuishi Y., Taguchi K., Kawatani Y., Shibata T., Nukiwa T., Aburatani H., Yamamoto M., Motohashi H. Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Cancer Cell. 2012;22:66–79. doi: 10.1016/j.ccr.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 96.Patra K.C., Hay N. The pentose phosphate pathway and cancer. Trends Biochem. Sci. 2014;39:347–354. doi: 10.1016/j.tibs.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ahn C.S., Metallo C.M. Mitochondria as biosynthetic factories for cancer proliferation. Cancer Metab. 2015;3:1. doi: 10.1186/s40170-015-0128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mandal P.K., Seiler A., Perisic T., Kölle P., Banjac Canak A., Förster H., Weiss N., Kremmer E., Lieberman M.W., Bannai S., et al. System x(c)- and thioredoxin reductase 1 cooperatively rescue glutathione deficiency. J. Biol. Chem. 2010;285:22244–22253. doi: 10.1074/jbc.M110.121327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Soula M., Weber R.A., Zilka O., Alwaseem H., La K., Yen F., Molina H., Garcia-Bermudez J., Pratt D.A., Birsoy K. Metabolic determinants of cancer cell sensitivity to canonical ferroptosis inducers. Nat. Chem. Biol. 2020;16:1351–1360. doi: 10.1038/s41589-020-0613-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cho H.Y., Gladwell W., Wang X., Chorley B., Bell D., Reddy S.P., Kleeberger S.R. Nrf2-regulated PPAR{gamma} expression is critical to protection against acute lung injury in mice. Am. J. Respir. Crit. Care Med. 2010;182:170–182. doi: 10.1164/rccm.200907-1047OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Huang J., Tabbi-Anneni I., Gunda V., Wang L. Transcription factor Nrf2 regulates SHP and lipogenic gene expression in hepatic lipid metabolism. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;299:G1211–G1221. doi: 10.1152/ajpgi.00322.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lewerenz J., Hewett S.J., Huang Y., Lambros M., Gout P.W., Kalivas P.W., Massie A., Smolders I., Methner A., Pergande M., et al. The cystine/glutamate antiporter system x(c)(-) in health and disease: From molecular mechanisms to novel therapeutic opportunities. Antioxid. Redox Signal. 2013;18:522–555. doi: 10.1089/ars.2011.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.He F., Antonucci L., Karin M. NRF2 as a regulator of cell metabolism and inflammation in cancer. Carcinogenesis. 2020;41:405–416. doi: 10.1093/carcin/bgaa039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Romero R., Sayin V.I., Davidson S.M., Bauer M.R., Singh S.X., LeBoeuf S.E., Karakousi T.R., Ellis D.C., Bhutkar A., Sánchez-Rivera F.J., et al. Keap1 loss promotes Kras-driven lung cancer and results in dependence on glutaminolysis. Nat. Med. 2017;23:1362–1368. doi: 10.1038/nm.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang H., Cheng Y., Mao C., Liu S., Xiao D., Huang J., Tao Y. Emerging mechanisms and targeted therapy of ferroptosis in cancer. Mol. Ther. 2021;29:2185–2208. doi: 10.1016/j.ymthe.2021.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Skouta R., Dixon S.J., Wang J., Dunn D.E., Orman M., Shimada K., Rosenberg P.A., Lo D.C., Weinberg J.M., Linkermann A., et al. Ferrostatins inhibit oxidative lipid damage and cell death in diverse disease models. J. Am. Chem. Soc. 2014;136:4551–4556. doi: 10.1021/ja411006a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yu Y., Xie Y., Cao L., Yang L., Yang M., Lotze M.T., Zeh H.J., Kang R., Tang D. The ferroptosis inducer erastin enhances sensitivity of acute myeloid leukemia cells to chemotherapeutic agents. Mol. Cell Oncol. 2015;2:e1054549. doi: 10.1080/23723556.2015.1054549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yang L., Wang H., Yang X., Wu Q., An P., Jin X., Liu W., Huang X., Li Y., Yan S., et al. Auranofin mitigates systemic iron overload and induces ferroptosis via distinct mechanisms. Signal Transduct. Target. Ther. 2020;5:138. doi: 10.1038/s41392-020-00253-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dixon S.J., Patel D.N., Welsch M., Skouta R., Lee E.D., Hayano M., Thomas A.G., Gleason C.E., Tatonetti N.P., Slusher B.S., et al. Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. eLife. 2014;3:e02523. doi: 10.7554/eLife.02523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Guo J., Xu B., Han Q., Zhou H., Xia Y., Gong C., Dai X., Li Z., Wu G. Ferroptosis: A Novel Anti-tumor Action for Cisplatin. Cancer Res. Treat. 2018;50:445–460. doi: 10.4143/crt.2016.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Notarnicola M., Altomare D.F., Correale M., Ruggieri E., D’Attoma B., Mastrosimini A., Guerra V., Caruso M.G. Serum lipid profile in colorectal cancer patients with and without synchronous distant metastases. Oncology. 2005;68:371–374. doi: 10.1159/000086977. [DOI] [PubMed] [Google Scholar]
- 112.Chen X., Kang R., Kroemer G., Tang D. Broadening horizons: The role of ferroptosis in cancer. Nat. Rev. Clin. Oncol. 2021;18:280–296. doi: 10.1038/s41571-020-00462-0. [DOI] [PubMed] [Google Scholar]
- 113.Dodson M., Castro-Portuguez R., Zhang D.D. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019;23:101107. doi: 10.1016/j.redox.2019.101107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wood E.R., Truesdale A.T., McDonald O.B., Yuan D., Hassell A., Dickerson S.H., Ellis B., Pennisi C., Horne E., Lackey K., et al. A unique structure for epidermal growth factor receptor bound to GW572016 (Lapatinib): Relationships among protein conformation, inhibitor off-rate, and receptor activity in tumor cells. Cancer Res. 2004;64:6652–6659. doi: 10.1158/0008-5472.CAN-04-1168. [DOI] [PubMed] [Google Scholar]
- 115.Haynes R.K., Cheu K.W., N’Da D., Coghi P., Monti D. Considerations on the mechanism of action of artemisinin antimalarials: Part 1--the ‘carbon radical’ and ‘heme’ hypotheses. Infect. Disord. Drug Targets. 2013;13:217–277. doi: 10.2174/1871526513666131129155708. [DOI] [PubMed] [Google Scholar]
- 116.Satoh H., Moriguchi T., Takai J., Ebina M., Yamamoto M. Nrf2 prevents initiation but accelerates progression through the Kras signaling pathway during lung carcinogenesis. Cancer Res. 2013;73:4158–4168. doi: 10.1158/0008-5472.CAN-12-4499. [DOI] [PubMed] [Google Scholar]
- 117.Tao S., Rojo de la Vega M., Chapman E., Ooi A., Zhang D.D. The effects of NRF2 modulation on the initiation and progression of chemically and genetically induced lung cancer. Mol. Carcinog. 2018;57:182–192. doi: 10.1002/mc.22745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.DeNicola G.M., Karreth F.A., Humpton T.J., Gopinathan A., Wei C., Frese K., Mangal D., Yu K.H., Yeo C.J., Calhoun E.S., et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475:106–109. doi: 10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang H., Liu X., Long M., Huang Y., Zhang L., Zhang R., Zheng Y., Liao X., Wang Y., Liao Q., et al. NRF2 activation by antioxidant antidiabetic agents accelerates tumor metastasis. Sci. Transl. Med. 2016;8:334–351. doi: 10.1126/scitranslmed.aad6095. [DOI] [PubMed] [Google Scholar]
- 120.Padmanabhan B., Tong K.I., Ohta T., Nakamura Y., Scharlock M., Ohtsuji M., Kang M.I., Kobayashi A., Yokoyama S., Yamamoto M. Structural basis for defects of Keap1 activity provoked by its point mutations in lung cancer. Mol. Cell. 2006;21:689–700. doi: 10.1016/j.molcel.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 121.Singh A., Misra V., Thimmulappa R.K., Lee H., Ames S., Hoque M.O., Herman J.G., Baylin S.B., Sidransky D., Gabrielson E., et al. Dysfunctional KEAP1-NRF2 interaction in non-small-cell lung cancer. PLoS Med. 2006;3:e420. doi: 10.1371/journal.pmed.0030420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tang K., Chen Q., Liu Y., Wang L., Lu W. Combination of Metformin and Sorafenib Induces Ferroptosis of Hepatocellular Carcinoma Through p62-Keap1-Nrf2 Pathway. J. Cancer. 2022;13:3234–3243. doi: 10.7150/jca.76618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gao A.M., Zhang X.Y., Ke Z.P. Apigenin sensitizes BEL-7402/ADM cells to doxorubicin through inhibiting miR-101/Nrf2 pathway. Oncotarget. 2017;8:82085–82091. doi: 10.18632/oncotarget.18294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yang W.S., SriRamaratnam R., Welsch M.E., Shimada K., Skouta R., Viswanathan V.S., Cheah J.H., Clemons P.A., Shamji A.F., Clish C.B., et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317–331. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chen C., Xie B., Li Z., Chen L., Chen Y., Zhou J., Ju S., Zhou Y., Zhang X., Zhuo W., et al. Fascin enhances the vulnerability of breast cancer to erastin-induced ferroptosis. Cell Death Dis. 2022;13:150. doi: 10.1038/s41419-022-04579-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Feng S., Li Y., Huang H., Huang H., Duan Y., Yuan Z., Zhu W., Mei Z., Luo L., Yan P. Isoorientin reverses lung cancer drug resistance by promoting ferroptosis via the SIRT6/Nrf2/GPX4 signaling pathway. Eur. J. Pharmacol. 2023;954:175853. doi: 10.1016/j.ejphar.2023.175853. [DOI] [PubMed] [Google Scholar]
- 127.Hsieh C.H., Hsieh H.C., Shih F.S., Wang P.W., Yang L.X., Shieh D.B., Wang Y.C. An innovative NRF2 nano-modulator induces lung cancer ferroptosis and elicits an immunostimulatory tumor microenvironment. Theranostics. 2021;11:7072–7091. doi: 10.7150/thno.57803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Liu X., Peng X., Cen S., Yang C., Ma Z., Shi X. Wogonin induces ferroptosis in pancreatic cancer cells by inhibiting the Nrf2/GPX4 axis. Front. Pharmacol. 2023;14:1129662. doi: 10.3389/fphar.2023.1129662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gout P.W., Buckley A.R., Simms C.R., Bruchovsky N. Sulfasalazine, a potent suppressor of lymphoma growth by inhibition of the x(c)-cystine transporter: A new action for an old drug. Leukemia. 2001;15:1633–1640. doi: 10.1038/sj.leu.2402238. [DOI] [PubMed] [Google Scholar]
- 130.Wahl C., Liptay S., Adler G., Schmid R.M. Sulfasalazine: A potent and specific inhibitor of nuclear factor kappa B. J. Clin. Investig. 1998;101:1163–1174. doi: 10.1172/JCI992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jing S., Lu Y., Zhang J., Ren Y., Mo Y., Liu D., Duan L., Yuan Z., Wang C., Wang Q. Levistilide a Induces Ferroptosis by Activating the Nrf2/HO-1 Signaling Pathway in Breast Cancer Cells. Drug Des. Devel Ther. 2022;16:2981–2993. doi: 10.2147/DDDT.S374328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wei R., Zhao Y., Wang J., Yang X., Li S., Wang Y., Yang X., Fei J., Hao X., Zhao Y., et al. Tagitinin C induces ferroptosis through PERK-Nrf2-HO-1 signaling pathway in colorectal cancer cells. Int. J. Biol. Sci. 2021;17:2703–2717. doi: 10.7150/ijbs.59404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Takaya K., Suzuki T., Motohashi H., Onodera K., Satomi S., Kensler T.W., Yamamoto M. Validation of the multiple sensor mechanism of the Keap1-Nrf2 system. Free Radic. Biol. Med. 2012;53:817–827. doi: 10.1016/j.freeradbiomed.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang B., Zhai M., Li B., Liu Z., Li K., Jiang L., Zhang M., Yi W., Yang J., Yi D., et al. Honokiol Ameliorates Myocardial Ischemia/Reperfusion Injury in Type 1 Diabetic Rats by Reducing Oxidative Stress and Apoptosis through Activating the SIRT1-Nrf2 Signaling Pathway. Oxid. Med. Cell Longev. 2018;2018:3159801. doi: 10.1155/2018/3159801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Xiao X., Lu Z., Lin V., May A., Shaw D.H., Wang Z., Che B., Tran K., Du H., Shaw P.X. MicroRNA miR-24-3p Reduces Apoptosis and Regulates Keap1-Nrf2 Pathway in Mouse Cardiomyocytes Responding to Ischemia/Reperfusion Injury. Oxid. Med. Cell Longev. 2018;2018:7042105. doi: 10.1155/2018/7042105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Park Y.H., Park H.P., Kim E., Lee H., Hwang J.W., Jeon Y.T., Lim Y.J. The antioxidant effect of preischemic dexmedetomidine in a rat model: Increased expression of Nrf2/HO-1 via the PKC pathway. Braz. J. Anesthesiol. 2023;73:177–185. doi: 10.1016/j.bjane.2021.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hu Q., Zuo T., Deng L., Chen S., Yu W., Liu S., Liu J., Wang X., Fan X., Dong Z. β-Caryophyllene suppresses ferroptosis induced by cerebral ischemia reperfusion via activation of the NRF2/HO-1 signaling pathway in MCAO/R rats. Phytomedicine. 2022;102:154112. doi: 10.1016/j.phymed.2022.154112. [DOI] [PubMed] [Google Scholar]
- 138.Chen J., Zhang J., Chen T., Bao S., Li J., Wei H., Hu X., Liang Y., Liu F., Yan S. Xiaojianzhong decoction attenuates gastric mucosal injury by activating the p62/Keap1/Nrf2 signaling pathway to inhibit ferroptosis. Biomed. Pharmacother. 2022;155:113631. doi: 10.1016/j.biopha.2022.113631. [DOI] [PubMed] [Google Scholar]
- 139.Cao T.H., Jin S.G., Fei D.S., Kang K., Jiang L., Lian Z.Y., Pan S.H., Zhao M.R., Zhao M.Y. Artesunate Protects Against Sepsis-Induced Lung Injury Via Heme Oxygenase-1 Modulation. Inflammation. 2016;39:651–662. doi: 10.1007/s10753-015-0290-2. [DOI] [PubMed] [Google Scholar]
- 140.Civantos E., Bosch E., Ramirez E., Zhenyukh O., Egido J., Lorenzo O., Mas S. Sitagliptin ameliorates oxidative stress in experimental diabetic nephropathy by diminishing the miR-200a/Keap-1/Nrf2 antioxidant pathway. Diabetes Metab. Syndr. Obes. 2017;10:207–222. doi: 10.2147/DMSO.S132537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 142.EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 143.Feng H., Schorpp K., Jin J., Yozwiak C.E., Hoffstrom B.G., Decker A.M., Rajbhandari P., Stokes M.E., Bender H.G., Csuka J.M., et al. Transferrin Receptor Is a Specific Ferroptosis Marker. Cell Rep. 2020;30:3411–3423. doi: 10.1016/j.celrep.2020.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jin Y., Yang R., Ding J., Zhu F., Zhu C., Xu Q., Cai J. KAT6A is associated with sorafenib resistance and contributes to progression of hepatocellular carcinoma by targeting YAP. Biochem. Biophys. Res. Commun. 2021;585:185–190. doi: 10.1016/j.bbrc.2021.09.009. [DOI] [PubMed] [Google Scholar]
- 145.Li Z.J., Dai H.Q., Huang X.W., Feng J., Deng J.H., Wang Z.X., Yang X.M., Liu Y.J., Wu Y., Chen P.H., et al. Artesunate synergizes with sorafenib to induce ferroptosis in hepatocellular carcinoma. Acta Pharmacol. Sin. 2021;42:301–310. doi: 10.1038/s41401-020-0478-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bai L., Wang H., Wang A.H., Zhang L.Y., Bai J. MicroRNA-532 and microRNA-3064 inhibit cell proliferation and invasion by acting as direct regulators of human telomerase reverse transcriptase in ovarian cancer. PLoS ONE. 2017;12:e0173912. doi: 10.1371/journal.pone.0173912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Harbeck N., Gnant M. Breast cancer. Lancet. 2017;389:1134–1150. doi: 10.1016/S0140-6736(16)31891-8. [DOI] [PubMed] [Google Scholar]
- 148.Hangauer M.J., Viswanathan V.S., Ryan M.J., Bole D., Eaton J.K., Matov A., Galeas J., Dhruv H.D., Berens M.E., Schreiber S.L., et al. Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition. Nature. 2017;551:247–250. doi: 10.1038/nature24297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ma S., Dielschneider R.F., Henson E.S., Xiao W., Choquette T.R., Blankstein A.R., Chen Y., Gibson S.B. Ferroptosis and autophagy induced cell death occur independently after siramesine and lapatinib treatment in breast cancer cells. PLoS ONE. 2017;12:e0182921. doi: 10.1371/journal.pone.0182921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ma S., Henson E.S., Chen Y., Gibson S.B. Ferroptosis is induced following siramesine and lapatinib treatment of breast cancer cells. Cell Death Dis. 2016;7:e2307. doi: 10.1038/cddis.2016.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yang Y., Zhang Y., Wang L., Lee S. Levistolide A Induces Apoptosis via ROS-Mediated ER Stress Pathway in Colon Cancer Cells. Cell Physiol. Biochem. 2017;42:929–938. doi: 10.1159/000478647. [DOI] [PubMed] [Google Scholar]
- 152.Cao M., Chen W. Epidemiology of lung cancer in China. Thorac. Cancer. 2019;10:3–7. doi: 10.1111/1759-7714.12916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Min Y., Mao C.Q., Chen S., Ma G., Wang J., Liu Y. Combating the drug resistance of cisplatin using a platinum prodrug based delivery system. Angew. Chem. Int. Ed. Engl. 2012;51:6742–6747. doi: 10.1002/anie.201201562. [DOI] [PubMed] [Google Scholar]
- 154.Zou Y., Wang X., Khan A., Wang P., Liu Y., Alsaedi A., Hayat T., Wang X. Environmental Remediation and Application of Nanoscale Zero-Valent Iron and Its Composites for the Removal of Heavy Metal Ions: A Review. Environ. Sci. Technol. 2016;50:7290–7304. doi: 10.1021/acs.est.6b01897. [DOI] [PubMed] [Google Scholar]
- 155.Yang L.X., Wu Y.N., Wang P.W., Huang K.J., Su W.C., Shieh D.B. Silver-coated zero-valent iron nanoparticles enhance cancer therapy in mice through lysosome-dependent dual programed cell death pathways: Triggering simultaneous apoptosis and autophagy only in cancerous cells. J. Mater. Chem. B. 2020;8:4122–4131. doi: 10.1039/C9TB01477B. [DOI] [PubMed] [Google Scholar]
- 156.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 157.Rahib L., Smith B.D., Aizenberg R., Rosenzweig A.B., Fleshman J.M., Matrisian L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 158.Liu X.Q., Jiang L., Li Y.Y., Huang Y.B., Hu X.R., Zhu W., Wang X., Wu Y.G., Meng X.M., Qi X.M. Wogonin protects glomerular podocytes by targeting Bcl-2-mediated autophagy and apoptosis in diabetic kidney disease. Acta Pharmacol. Sin. 2022;43:96–110. doi: 10.1038/s41401-021-00721-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Guan J., Lo M., Dockery P., Mahon S., Karp C.M., Buckley A.R., Lam S., Gout P.W., Wang Y.Z. The xc-cystine/glutamate antiporter as a potential therapeutic target for small-cell lung cancer: Use of sulfasalazine. Cancer Chemother. Pharmacol. 2009;64:463–472. doi: 10.1007/s00280-008-0894-4. [DOI] [PubMed] [Google Scholar]
- 160.Okazaki S., Shintani S., Hirata Y., Suina K., Semba T., Yamasaki J., Umene K., Ishikawa M., Saya H., Nagano O. Synthetic lethality of the ALDH3A1 inhibitor dyclonine and xCT inhibitors in glutathione deficiency-resistant cancer cells. Oncotarget. 2018;9:33832–33843. doi: 10.18632/oncotarget.26112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ranti I., Wahyuningsih M.S.H., Wirohadidjojo Y.W. The antifibrotic effect of isolate tagitinin C from tithonia diversifolia (Hemsley) A. Gray on keloid fibroblast cell. Pan Afr. Med. J. 2018;30:264. doi: 10.11604/pamj.2018.30.264.9994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Liao M.H., Lin W.C., Wen H.C., Pu H.F. Tithonia diversifolia and its main active component tagitinin C induce survivin inhibition and G2/M arrest in human malignant glioblastoma cells. Fitoterapia. 2011;82:331–341. doi: 10.1016/j.fitote.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 163.Zhao L., Dong J., Hu Z., Li S., Su X., Zhang J., Yin Y., Xu T., Zhang Z., Chen H. Anti-TMV activity and functional mechanisms of two sesquiterpenoids isolated from Tithonia diversifolia. Pestic. Biochem. Physiol. 2017;140:24–29. doi: 10.1016/j.pestbp.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 164.Gonçalves-Santos E., Vilas-Boas D.F., Diniz L.F., Veloso M.P., Mazzeti A.L., Rodrigues M.R., Oliveira C.M., Fernandes V.H.C., Novaes R.D., Chagas-Paula D.A., et al. Sesquiterpene lactone potentiates the immunomodulatory, antiparasitic and cardioprotective effects on anti-Trypanosoma cruzi specific chemotherapy. Int. Immunopharmacol. 2019;77:105961. doi: 10.1016/j.intimp.2019.105961. [DOI] [PubMed] [Google Scholar]
- 165.Kasarskis E.J., Tandon L., Lovell M.A., Ehmann W.D. Aluminum, calcium, and iron in the spinal cord of patients with sporadic amyotrophic lateral sclerosis using laser microprobe mass spectroscopy: A preliminary study. J. Neurol. Sci. 1995;130:203–208. doi: 10.1016/0022-510X(95)00037-3. [DOI] [PubMed] [Google Scholar]
- 166.Kaur D., Yantiri F., Rajagopalan S., Kumar J., Mo J.Q., Boonplueang R., Viswanath V., Jacobs R., Yang L., Beal M.F., et al. Genetic or pharmacological iron chelation prevents MPTP-induced neurotoxicity in vivo: A novel therapy for Parkinson’s disease. Neuron. 2003;37:899–909. doi: 10.1016/S0896-6273(03)00126-0. [DOI] [PubMed] [Google Scholar]
- 167.Zhu W., Xie W., Pan T., Xu P., Fridkin M., Zheng H., Jankovic J., Youdim M.B., Le W. Prevention and restoration of lactacystin-induced nigrostriatal dopamine neuron degeneration by novel brain-permeable iron chelators. FASEB J. 2007;21:3835–3844. doi: 10.1096/fj.07-8386com. [DOI] [PubMed] [Google Scholar]
- 168.Buijs M., Doan N.T., van Rooden S., Versluis M.J., van Lew B., Milles J., van der Grond J., van Buchem M.A. In vivo assessment of iron content of the cerebral cortex in healthy aging using 7-Tesla T2*-weighted phase imaging. Neurobiol. Aging. 2017;53:20–26. doi: 10.1016/j.neurobiolaging.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 169.Lei P., Ayton S., Finkelstein D.I., Spoerri L., Ciccotosto G.D., Wright D.K., Wong B.X., Adlard P.A., Cherny R.A., Lam L.Q., et al. Tau deficiency induces parkinsonism with dementia by impairing APP-mediated iron export. Nat. Med. 2012;18:291–295. doi: 10.1038/nm.2613. [DOI] [PubMed] [Google Scholar]
- 170.Li L.B., Chai R., Zhang S., Xu S.F., Zhang Y.H., Li H.L., Fan Y.G., Guo C. Iron Exposure and the Cellular Mechanisms Linked to Neuron Degeneration in Adult Mice. Cells. 2019;8:198. doi: 10.3390/cells8020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Connor J.R., Menzies S.L., St Martin S.M., Mufson E.J. A histochemical study of iron, transferrin, and ferritin in Alzheimer’s diseased brains. J. Neurosci. Res. 1992;31:75–83. doi: 10.1002/jnr.490310111. [DOI] [PubMed] [Google Scholar]
- 172.Spotorno N., Acosta-Cabronero J., Stomrud E., Lampinen B., Strandberg O.T., van Westen D., Hansson O. Relationship between cortical iron and tau aggregation in Alzheimer’s disease. Brain. 2020;143:1341–1349. doi: 10.1093/brain/awaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Becerril-Ortega J., Bordji K., Fréret T., Rush T., Buisson A. Iron overload accelerates neuronal amyloid-β production and cognitive impairment in transgenic mice model of Alzheimer’s disease. Neurobiol. Aging. 2014;35:2288–2301. doi: 10.1016/j.neurobiolaging.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 174.Zhang Y.H., Wang D.W., Xu S.F., Zhang S., Fan Y.G., Yang Y.Y., Guo S.Q., Wang S., Guo T., Wang Z.Y., et al. α-Lipoic acid improves abnormal behavior by mitigation of oxidative stress, inflammation, ferroptosis, and tauopathy in P301S Tau transgenic mice. Redox Biol. 2018;14:535–548. doi: 10.1016/j.redox.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ayton S., Lei P., Duce J.A., Wong B.X., Sedjahtera A., Adlard P.A., Bush A.I., Finkelstein D.I. Ceruloplasmin dysfunction and therapeutic potential for Parkinson disease. Ann. Neurol. 2013;73:554–559. doi: 10.1002/ana.23817. [DOI] [PubMed] [Google Scholar]
- 176.Dexter D.T., Wells F.R., Lees A.J., Agid F., Agid Y., Jenner P., Marsden C.D. Increased nigral iron content and alterations in other metal ions occurring in brain in Parkinson’s disease. J. Neurochem. 1989;52:1830–1836. doi: 10.1111/j.1471-4159.1989.tb07264.x. [DOI] [PubMed] [Google Scholar]
- 177.Davies K.M., Hare D.J., Cottam V., Chen N., Hilgers L., Halliday G., Mercer J.F., Double K.L. Localization of copper and copper transporters in the human brain. Metallomics. 2013;5:43–51. doi: 10.1039/C2MT20151H. [DOI] [PubMed] [Google Scholar]
- 178.Pearce R.K., Owen A., Daniel S., Jenner P., Marsden C.D. Alterations in the distribution of glutathione in the substantia nigra in Parkinson’s disease. J. Neural. Transm. 1997;104:661–677. doi: 10.1007/BF01291884. [DOI] [PubMed] [Google Scholar]
- 179.Bender A., Krishnan K.J., Morris C.M., Taylor G.A., Reeve A.K., Perry R.H., Jaros E., Hersheson J.S., Betts J., Klopstock T., et al. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat. Genet. 2006;38:515–517. doi: 10.1038/ng1769. [DOI] [PubMed] [Google Scholar]
- 180.Devos D., Moreau C., Devedjian J.C., Kluza J., Petrault M., Laloux C., Jonneaux A., Ryckewaert G., Garçon G., Rouaix N., et al. Targeting chelatable iron as a therapeutic modality in Parkinson’s disease. Antioxid. Redox Signal. 2014;21:195–210. doi: 10.1089/ars.2013.5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Park T.J., Park J.H., Lee G.S., Lee J.Y., Shin J.H., Kim M.W., Kim Y.S., Kim J.Y., Oh K.J., Han B.S., et al. Quantitative proteomic analyses reveal that GPX4 downregulation during myocardial infarction contributes to ferroptosis in cardiomyocytes. Cell Death Dis. 2019;10:835. doi: 10.1038/s41419-019-2061-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Song Y., Wang B., Zhu X., Hu J., Sun J., Xuan J., Ge Z. Human umbilical cord blood-derived MSCs exosome attenuate myocardial injury by inhibiting ferroptosis in acute myocardial infarction mice. Cell Biol. Toxicol. 2021;37:51–64. doi: 10.1007/s10565-020-09530-8. [DOI] [PubMed] [Google Scholar]
- 183.Jin R., Yang R., Cui C., Zhang H., Cai J., Geng B., Chen Z. Ferroptosis due to Cystathionine γ Lyase/Hydrogen Sulfide Downregulation Under High Hydrostatic Pressure Exacerbates VSMC Dysfunction. Front. Cell Dev. Biol. 2022;10:829316. doi: 10.3389/fcell.2022.829316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wang C., Yuan W., Hu A., Lin J., Xia Z., Yang C.F., Li Y., Zhang Z. Dexmedetomidine alleviated sepsis-induced myocardial ferroptosis and septic heart injury. Mol. Med. Rep. 2020;22:175–184. doi: 10.3892/mmr.2020.11114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Dai C., Kong B., Qin T., Xiao Z., Fang J., Gong Y., Zhu J., Liu Q., Fu H., Meng H., et al. Inhibition of ferroptosis reduces susceptibility to frequent excessive alcohol consumption-induced atrial fibrillation. Toxicology. 2022;465:153055. doi: 10.1016/j.tox.2021.153055. [DOI] [PubMed] [Google Scholar]
- 186.Menon A.V., Liu J., Tsai H.P., Zeng L., Yang S., Asnani A., Kim J. Excess heme upregulates heme oxygenase 1 and promotes cardiac ferroptosis in mice with sickle cell disease. Blood. 2022;139:936–941. doi: 10.1182/blood.2020008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Liu P., Feng Y., Li H., Chen X., Wang G., Xu S., Li Y., Zhao L. Ferrostatin-1 alleviates lipopolysaccharide-induced acute lung injury via inhibiting ferroptosis. Cell Mol. Biol. Lett. 2020;25:10. doi: 10.1186/s11658-020-00205-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Liang N.N., Zhao Y., Guo Y.Y., Zhang Z.H., Gao L., Yu D.X., Xu D.X., Xu S. Mitochondria-derived reactive oxygen species are involved in renal cell ferroptosis during lipopolysaccharide-induced acute kidney injury. Int. Immunopharmacol. 2022;107:108687. doi: 10.1016/j.intimp.2022.108687. [DOI] [PubMed] [Google Scholar]
- 189.Zager R.A., Foerder C.A. Effects of inorganic iron and myoglobin on in vitro proximal tubular lipid peroxidation and cytotoxicity. J. Clin. Investig. 1992;89:989–995. doi: 10.1172/JCI115682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Martin-Sanchez D., Ruiz-Andres O., Poveda J., Carrasco S., Cannata-Ortiz P., Sanchez-Niño M.D., Ruiz Ortega M., Egido J., Linkermann A., Ortiz A., et al. Ferroptosis, but Not Necroptosis, Is Important in Nephrotoxic Folic Acid-Induced AKI. J. Am. Soc. Nephrol. 2017;28:218–229. doi: 10.1681/ASN.2015121376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Paller M.S. Hemoglobin- and myoglobin-induced acute renal failure in rats: Role of iron in nephrotoxicity. Am. J. Physiol. 1988;255:F539–F544. doi: 10.1152/ajprenal.1988.255.3.F539. [DOI] [PubMed] [Google Scholar]
- 192.Havrdova E., Giovannoni G., Gold R., Fox R.J., Kappos L., Phillips J.T., Okwuokenye M., Marantz J.L. Effect of delayed-release dimethyl fumarate on no evidence of disease activity in relapsing-remitting multiple sclerosis: Integrated analysis of the phase III DEFINE and CONFIRM studies. Eur. J. Neurol. 2017;24:726–733. doi: 10.1111/ene.13272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Cuadrado A. NRF2 in neurodegenerative diseases. Curr. Opin. Toxicol. 2016;1:46–53. doi: 10.1016/j.cotox.2016.09.004. [DOI] [Google Scholar]
- 194.Huang K., Gao X., Wei W. The crosstalk between Sirt1 and Keap1/Nrf2/ARE anti-oxidative pathway forms a positive feedback loop to inhibit FN and TGF-β1 expressions in rat glomerular mesangial cells. Exp. Cell Res. 2017;361:63–72. doi: 10.1016/j.yexcr.2017.09.042. [DOI] [PubMed] [Google Scholar]
- 195.Miao Y., Wan Q., Liu X., Wang Y., Luo Y., Liu D., Lin N., Zhou H., Zhong J. miR-503 Is Involved in the Protective Effect of Phase II Enzyme Inducer (CPDT) in Diabetic Cardiomyopathy via Nrf2/ARE Signaling Pathway. Biomed. Res. Int. 2017;2017:9167450. doi: 10.1155/2017/9167450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Li Y., Duan J.Z., He Q., Wang C.Q. miR-155 modulates high glucose-induced cardiac fibrosis via the Nrf2/HO-1 signaling pathway. Mol. Med. Rep. 2020;22:4003–4016. doi: 10.3892/mmr.2020.11495. [DOI] [PubMed] [Google Scholar]
- 197.Yu M., Liu Y., Zhang B., Shi Y., Cui L., Zhao X. Inhibiting microRNA-144 abates oxidative stress and reduces apoptosis in hearts of streptozotocin-induced diabetic mice. Cardiovasc. Pathol. 2015;24:375–381. doi: 10.1016/j.carpath.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 198.Zhang J., Cai W., Fan Z., Yang C., Wang W., Xiong M., Ma C., Yang J. MicroRNA-24 inhibits the oxidative stress induced by vascular injury by activating the Nrf2/Ho-1 signaling pathway. Atherosclerosis. 2019;290:9–18. doi: 10.1016/j.atherosclerosis.2019.08.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.