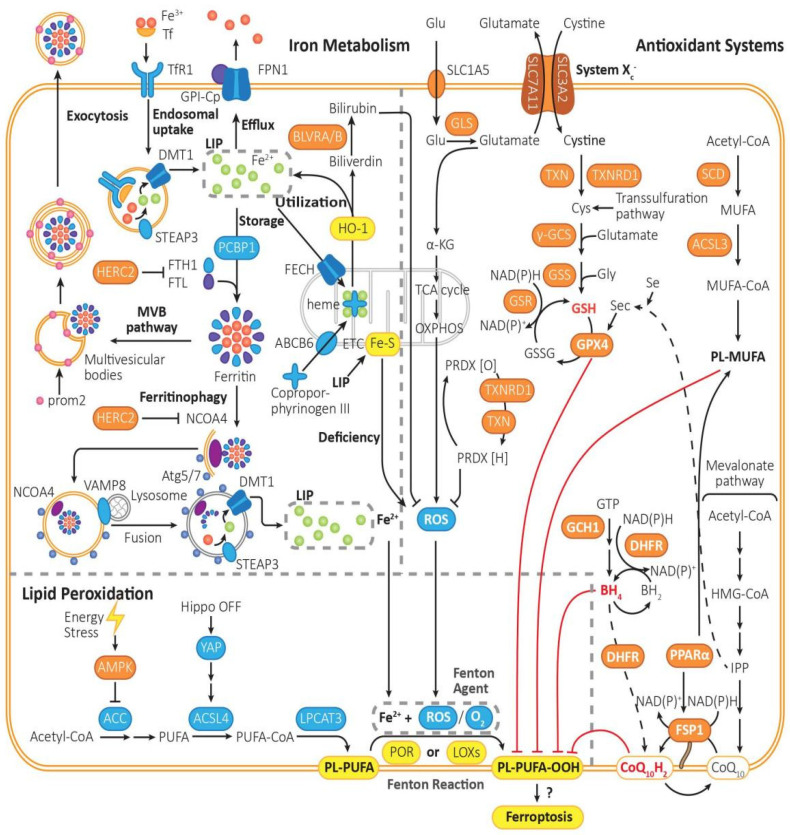
Figure 1.
The mechanism of ferroptosis. Ferroptosis is driven by iron-dependent lipid peroxidation, the reaction of PL-PUFA with Fe2+ and ROS under the catalysis of POR or LOXs. Ferroptosis is regulated by lipid metabolism, iron metabolism, and intracellular antioxidant system. (1) Lipid metabolism: PL-PUFA, the prime substrate for lipid peroxidation, is synthesized from Acetyl-CoA in multiple steps, which are catalyzed by ACC, ACSL4, and LPCAT3. Then, it is prone to be peroxidized to PL-PUFA-OOH, which triggers the followed reactions of ferroptosis, though the initiation mechanism is still not quite clear. The peroxidation process is mainly driven by Fenton reaction, or mediated by POR or LOXs. By activating AMPK, energy stress can inhibit the activity of ACC, thus turning on an energy stress-protective program against ferroptosis. (2) Iron metabolism: The peroxidation of PL-PUFA is driven by the labile iron pool and iron-dependent enzymes. Fe3+ is imported into cells by Tf through TfR1, then being reduced by STEAP3 to Fe2+, forming the labile iron pool. Excess Fe2+ can be expelled via FPN1, be stored in ferritin, which can be exported through MVB pathway or release Fe2+ through ferritinophagy, or be utilized to synthesize heme with coproporphyrinogen III through ABCB6 and FECH, or regenerated when heme is metabolized by HO-1. (3) Intracellular antioxidant system: There are mainly three pathways for removing PL-PUFA-OOHs. Cyst(e)ine/GSH/GPX4 axis: It is one of the most important pathways in ferroptosis inhibition. It requires uptake of cystine via system Xc−, reduction of cystine to cysteine, and biosynthesis of GSH. GPX4-mediated reduction of PL-PUFA-OOH to PL-PUFA-OH, and regeneration of GSH from GSSG. FSP1/CoQ10 axis: It functions independently of GPX4. CoQ10H2 can remove lipid peroxides, and then consume NADPH to regenerate CoQ10H2 by FSP1. GCH1/BH4/DHFR system: BH4 can be synthesized from GTP under the catalysis of GCH1, being converted into BH2 and clearing lipid peroxidants, and then regenerated by DHFR. Furthermore, saturated fatty acid can be transformed into PL-MUFA in multi-steps, which is the competing substrate against PL-PUFA and can suppress ferroptosis. Furthermore, PRDX can reduce the content of ROS, which can be generated from PRDX[O] through NADPH and TRX.