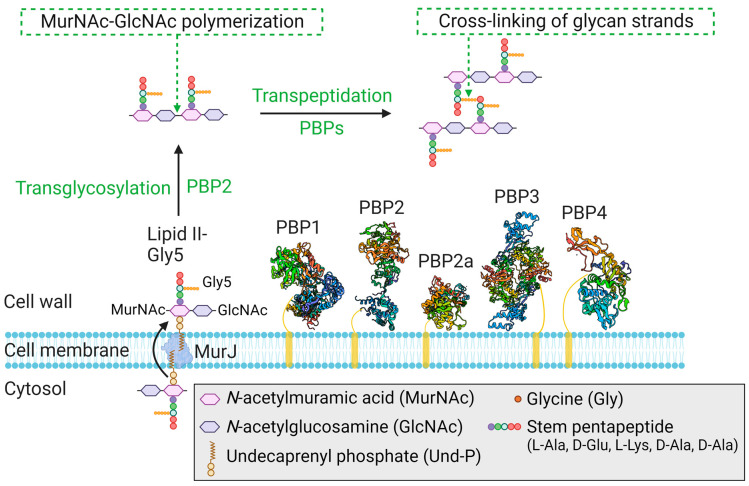
Figure 5.
Structural and organizational representation of PBPs that play a crucial role in the final stages of peptidoglycan synthesis in S. aureus. The crystal structures of PBP1 (5TRO), PBP2 (2OLU), PBP2a (1VQQ), PBP3 (3VSK), and PBP4 (6C39) are shown. Endogenous PBP1-4 and acquired PBP2a all have a TPase domain, while PBP2 is unique in also possessing a TGase domain. After flipping to the outer side of the cytoplasmic membrane by MurJ, the peptidoglycan precursor Lipid II-Gly5 can be polymerized by the TGase activity of PBP2, and the glycan strands are then cross-linked by the TPase activity of the PBPs [65].