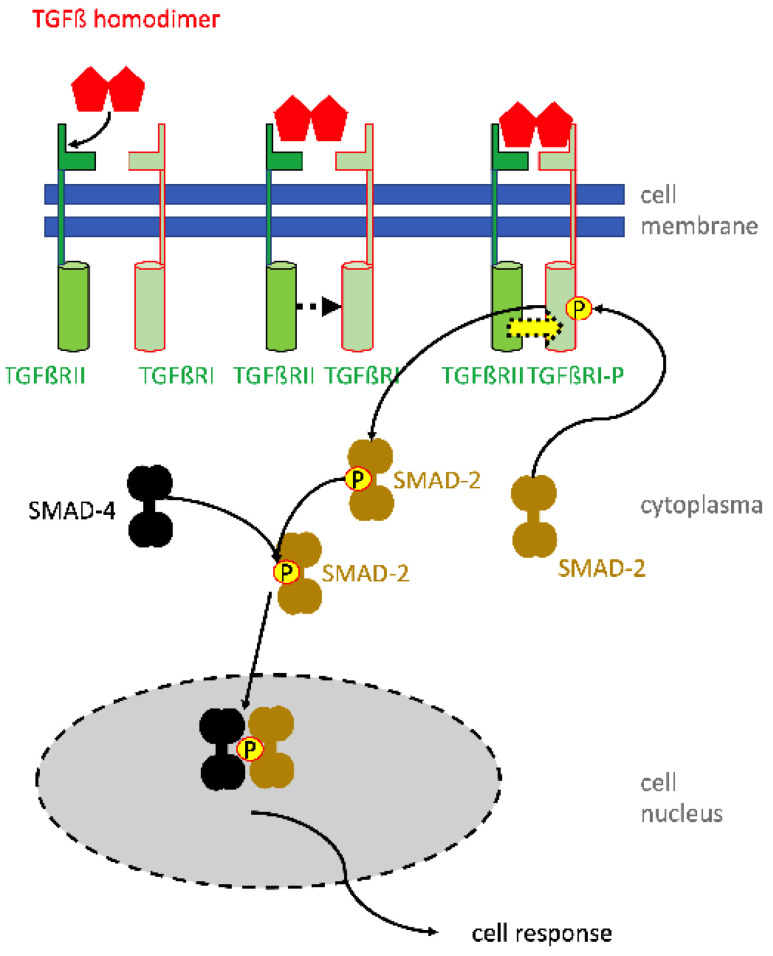
Figure 3.
Activation of cells by TGF-beta using the SMAD signaling pathway. The homodimer of TGF-beta binds first with low affinity to the TGF-beta receptor 2 (TGFbRII) (top left). This causes a conformational change facilitating an approximation of the TGFßRII molecule to TGFßRI (top middle). Adjoining TGFßRs form a high-affinity receptor binding TGFß at the binding epitopes of both receptor components (top right). The close heterodimeric receptor enables the intracellular kinase domain of TGFRII to phosphorylate and thereby activate the corresponding domain of TGFßRI (yellow arrow). Activated TGFßRI interacts with SMAD-2 (or SMAD-3) and phosphorylates SMAD-2 or -3 (below). Phosphorylated SMAD-2 then interacts with SMAD-4. The pSMAD-2/SMAD-4 complex translocates in the cell nucleus and there interacts with transcription initiation and elongation factors to activate the gene expression of the respective target genes. The TGF-mediated intracellular signaling via MAP-kinases (e.g., ERK, p38) and Pi3 kinase, regulating downstream AKT and TOR, are omitted in the graph, but play an important role in the anti-apoptotic action of TGF.