Figure 4. Residue-Pair Distance Fluctuations and Domain Cross-Talk for GRP94 in Different Glycosylation and Ligand Conditions.
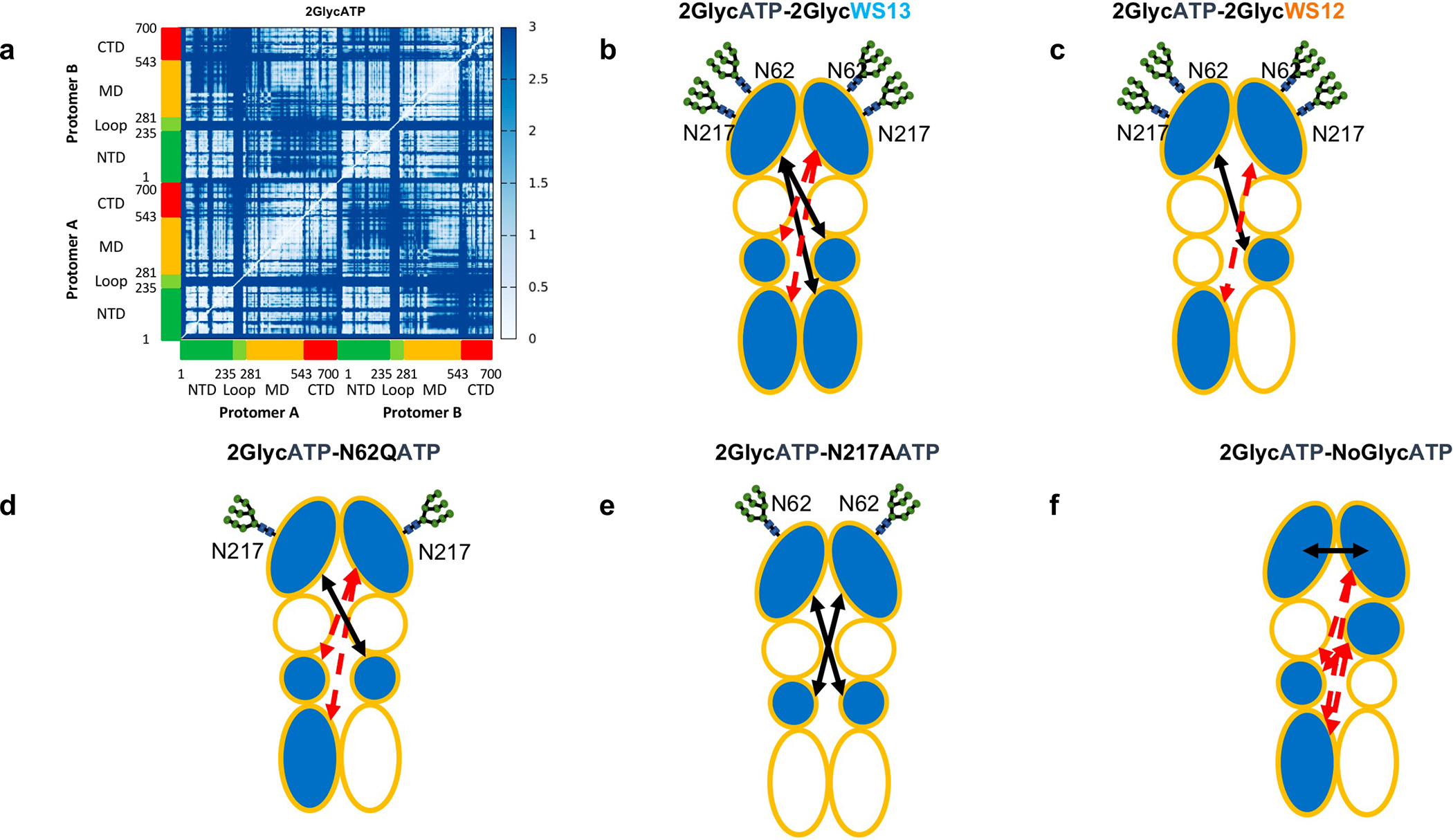
The internal dynamics of GRP94 characterized from the various trajectories.
(a) The original Distance Fluctuation matrix is reported for the fully-glycosylated ATP-bound state. The axes report the residue numbering and domain organisation, as depicted also in Figure 2. In this view, lighter pixels correspond to highly coordinated residue pairs, while darker ones report on low coordination pairs. The single original matrices for all 9 conditions, together with the difference matrices obtained by subtracting the DF matrix of a certain state from the reference one pertaining to 2GlycATP are reported in Supplementary Information.
(b-f) Domain based representations of the modulation of internal flexibility as a function of ligand-state and/or glycosylation state. This representation is a simplified graphical translation of the difference matrices reported in the Supplementary Information (Figures S8–S9). A certain domain is colored in light-blue if its coordination with other domains changes as a function of the condition. The variation in internal dynamics is evaluated as a difference for the DF of the system under exam from that of the fully glycosylated ATP-bound state, 2GlycATP. A black arrow indicates increased coordination (rigidity) between two domains with respect to the fully glycosylated ATP-bound state, 2GlycATP. A red, broken arrow indicates decreased coordination (flexibility) between two domains with respect to the fully glycosylated ATP-bound state, 2GlycATP. The various ligand states are reported in each subfigure.
