Abstract
Objectives:
To identify neonatal characteristics and 2-year neurodevelopmental outcomes associated with positive screening for risk of autism.
Study Design:
Nine university-affiliated neonatal intensive care units (NICUs) enrolled infants born at <30 weeks of gestation. Infants underwent the NICU Network Neurobehavioral Scale (NNNS) examination before discharge and the Bayley Scales of Infant and Toddler Development, Third Edition (Bayley-III), the Child Behavior Checklist (CBCL), and the Modified Checklist for Autism in Toddlers, revised with follow-up (M-CHAT-R/F) at two years corrected age. Generalized estimating equations examined associations between M-CHAT-R/F, neurobehavioral test results, and neonatal medical morbidities.
Results:
At two years corrected age, data were available for 466/744 enrolled infants without cerebral palsy. Infants with hypo-aroused NNNS profiles were more likely to screen M-CHAT-R/F positive (OR=2.76, 95% CI: 1.38, 5.54).). Infants with ≥ 2 medical morbidities also were more likely to screen positive (OR=2.65, 95% CI: 1.27, 5.54).). Children with positive M-CHAT-R/F scores had lower Bayley-III Cognitive (t (451)=5.43, p < .001, d=0.82), Language (t (53.49)= 7.82, p < .001, d=1.18), and Motor (t (451)=7.98, p < .001, d=1.21) composite scores and significantly higher CBCL Internalizing (t (457) −6.19, p < .001, d=−0.93) and Externalizing (t (57.87)=−5.62, p < .001, d=−0.84) scores.
Conclusions:
Positive M-CHAT-R/F screens at 2 years corrected age were associated with neonatal medical morbidities and neurobehavioral examinations as well as toddler developmental and behavioral outcomes. These findings demonstrate the potential utility of the M-CHAT-R/F as a global developmental screener in infants born very preterm, regardless of whether there is a later autism diagnosis.
Keywords: neurobehavior, medical conditions, very preterm, development, autism, screening
Infants born very preterm (<30 weeks of gestation) have 4 times the risk of autism compared with full-term infants(1). Although this risk appears to be perinatal in origin, most autism diagnoses are made during early childhood(2). Screening for autism is often conducted using either the Modified Checklist for Autism in Toddlers (M-CHAT)(3) or the M-CHAT, Revised with Follow-up (R/F), which has improved false positive and false negative rates (4). Prior studies have shown that positive M-CHAT screens were associated not only with autism, but also with behavioral and global developmental impairments in infants born preterm(3,5). For example, infants with birth weights ≤1500 grams who screened M-CHAT positive had higher internalizing scores on the Child Behavior Checklist (CBCL) at 24 months than infants who screened negative(5). Furthermore, among infants born at <28 weeks of gestation, positive M-CHAT results predicted lower Bayley Psychomotor and Mental Development Indices at 24 months(3). Early identification of infants at risk for positive M-CHAT screens could promote earlier intervention for infants born very preterm whether or not they develop autism. Predischarge assessments using the NICU Network Neurobehavioral Scale (NNNS)(6) have been shown to predict developmental outcomes in preterm and other at risk cohorts(7–10). For example, attention responses on the NNNS predict social attention in later infancy, as well as developmental outcomes at 12 months in infants at risk for autism(11,12). However, there are currently no studies assessing whether infant neurobehavioral regulation, as measured by the NNNS, is associated with later positive screens using the M-CHAT-R/F.
This study aimed to determine associations between positive M-CHAT-R/F screens at 24 months corrected age and high neurobehavioral risk scores on predischarge NNNS as well as medical morbidities in infants born very preterm. A second aim was to describe neurodevelopmental and behavioral characteristics associated with positive M-CHAT-R/F screens at 24 months corrected age in infants born very preterm.
METHODS
Infants (n = 704) enrolled in the NOVI study were recruited from nine university-affiliated NICUs from April 2014 to June 2016. These neonatal intensive care units (NICUs) participated in the Vermont Oxford Network and neonatal data for the current study was collected using standardized definitions and procedures developed by the Vermont Oxford Network. Inclusion criteria included: (1) birth at <30 weeks postmenstrual age; (2) parent ability to read and speak in English or Spanish; (3) residence within three hours of the NICU and the follow-up clinic. Exclusion criteria included: (1) major congenital anomalies, (2) maternal age <18 years, (3) maternal cognitive impairment, resulting in inability to provide informed consent. Parents were invited to participate in NOVI when their infant reached 31–32 weeks postmenstrual age, or when survival to discharge was determined likely by the attending neonatologist. Research staff obtained consent in accordance with individual site institutional review boards. All mothers provided written informed consent. Children were included in the present study if they were enrolled in NOVI and completed the 24-month corrected age follow-up at their enrollment institution.
Neonatal Measures
NICU Network Neurobehavioral Scale (NNNS)(6).
The NNNS is a 20–30-minute neurobehavioral exam conducted during the week prior to NICU discharge measuring primitive reflexes, tone, neurological integrity, behavioral functioning, and signs of stress/abstinence. Examiners were trained and certified to reliability by a NOVI NNNS trainer and exams were conducted prior to a scheduled feeding and/or routine care to maximize alertness and minimize interruption to NICU care routines and sleep. Individual items were converted to summary scores: attention, handling, self-regulation, arousal, excitability, lethargy, hypertonia, hypotonia, non-optimal reflexes, asymmetric reflexes, quality of movement, and stress abstinence. Table 1 (available online at jpeds.com) shows definitions of each summary score. Summary scores were converted to NNNS profiles, as published in previous work utilizing participants from the NOVI study(13).
Table 1.
NNNS Summary Scores
| NNNS domain | Description |
|---|---|
| Attention | Localize and track objects, faces, and voices |
| Handling | Strategies used to support attention |
| Regulation | Organize behavior in response to stimulation |
| Arousal | Degree of physiological and behavioral stimulation |
| Tone | Hyper and hypotonicity |
| Reflexes | Poor and asymmetric reflexes |
| Movement | Smoothness, maturity, lack of startles and tremors |
| Stress Abstinence | Number of stress signs observed (e.g., high pitched crying, gaze aversion, abnormal posture) |
Neonatal Medical Complications Index.
Medical complications in this validated index(14) include (1) severe brain injury, including parenchymal echodensity, periventricular leukomalacia, and moderate to severe ventriculomegaly; (2) severe retinopathy of prematurity; (3) bronchopulmonary dysplasia; (4) culture-positive sepsis or necrotizing enterocolitis. Each medical complication equates to one point and a cumulative index of medical complications was created by summing the presence of morbidities from the NICU course.
Outcome Measures
All outcome measures were administered at the follow up visit at 24 months corrected age.
Modified Checklist of Autism in Toddlers, Revised with Follow-Up (M-CHAT-R/F)(4).
The M-CHAT-R/F is a two-step, 20 item screener for autism risk that was administered by a research associate. Scores of 0–2 are considered low risk. For scores of 3–7, parents completed additional in-depth follow-up questions. Follow up scores of 2–7 are classified as medium risk and scores of 8–20 as high-risk for autism.
Bayley Scales of Infant and Toddler Development, 3rd edition (Bayley-III)(15).
The Bayley-III is a widely used developmental assessment yielding composite scores (standard scores) for Cognitive, Language, and Motor development. For this study, we used the three composite scores which have a mean of 100 with a standard deviation of 15. The Bayley-III was administered by licensed, experienced clinical psychologists.
Child Behavior Checklist (CBCL)(16).
The CBCL is a parent-report measure of child behavior problems. Parents rate 99 behaviors with resultant t-scores scores for Internalizing Problems, Externalizing Problems, and a Total Problems score. The Withdrawn subscale and the Pervasive Developmental Disorders (PDD) subscale were included in analyses as they are indicators of autism-related symptoms. The normative mean for each subscale is 50 (standard deviation = 10). A t-score of ≥ 64 is classified as a clinically significant problem.
Statistical Analyses
Latent profile analysis (LPA) was used to classify infants into mutually exclusive groups using the NNNS summary scores. The development of NNNS profiles utilized in the current paper with the NOVI study cohort has been detailed previously(13). Briefly, LPA uses maximum likelihood estimation to determine the optimal number of mutually exclusive profiles that exist in the data based on a specific set of variables(17) and in the case of the NNNS scores, a six-profile solution was identified(13). Infants in profiles 5 (hypo-aroused) and 6 (hyper-aroused) had scores that reflected the poorest functioning in multiple domains, with all 6 profiles being mutually exclusive (Figure 1)(13). Neurobehavioral risks were dichotomized in three ways in this study: (1) infants having “high neurobehavioral risk” (NNNS profiles 5–6) vs. “low neurobehavioral risk” (NNNS profiles 1–4) as previously reported(13), (2) infants having “hypo-aroused neurobehavior” (NNNS profile 5) vs. “low neurobehavioral risk” (profiles 1–4), and (3) infants having “hyper-aroused neurobehavior” (NNNS profile 6) vs. “low neurobehavioral risk” (profiles 1–4). Individual models were examined for hypo- and hyper-aroused neurobehavior to pinpoint whether a specific profile of atypical neurobehavior presents greater risk of a positive M-CHAT-R/F screen. The number of medical complications were dichotomized using ≥ 2 complications to indicate high medical risk and ≤ 1 indicating low medical risk, based on prior work(18). For M-CHAT-R/F, children who screened at medium and high risk were categorized into one group as a positive screen on the M-CHAT-R/F with all other children being considered a negative M-CHAT-R/F screen.
Figure 1:
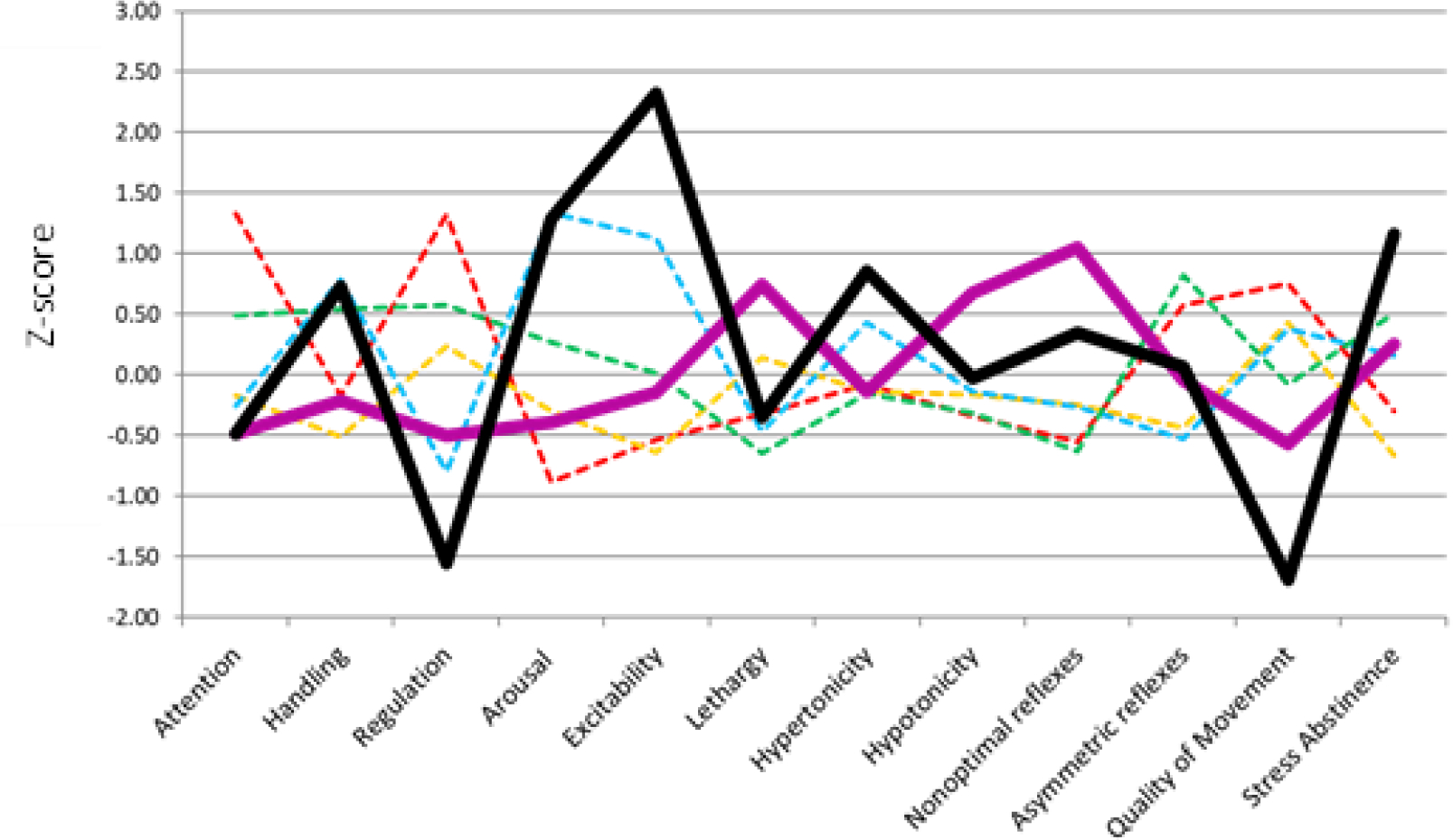
NNNS Profiles for the Cohort. Behaviors assessed on the NNNS are on the X-axis with individual lines showing developmental profiles identified by latent profile analysis.
Children with a cerebral palsy (CP) diagnosis (n = 85) were removed from the analyses to minimize bias given previous findings that children with motor, cognitive, and hearing impairments were significantly more likely to screen positive on the M-CHAT(19). Maternal and infant demographic characteristics were summarized in preparation for inclusion in generalized estimating equation (GEE) models. Race and ethnicity data were obtained via survey completed by mothers which were then combined and dichotomized into a minority group and non-minority group in line with previous work with this sample (10,13) which also allows us to account for small cell sizes across some groups. This dichotomous variable was included as a covariate in analyses to control for potential group differences in M-CHAT-R/F screening as the primary questions of interest surrounded the associations between neonatal neurobehavior, medical complications, and the screening results. To test aim one, GEE was conducted to determine the association between M-CHAT-R/F outcomes at 24 months with cumulative medical complications, NNNS profiles, and the interaction between medical complications and NNNS profiles. GEE analyses controlled for demographic variables, while nesting site and multi-birth families. To test aim two, independent samples t-tests were conducted comparing M-CHAT-R/F positive and negative screens on 2-year outcomes (Bayley-III composite scores and CBCL summary scores).
RESULTS
M-CHAT-R/F data were available for 466 participants at the two-year follow up, of whom 415 (89.1%) screened negative and 51 (10.9%) screened positive (medium-risk, n = 45; high-risk, n=6). These categories were combined due to the low sample size in the high-risk category. See Figure 2 (available online at jpeds.com) for a participant flowchart.
Figure 2:
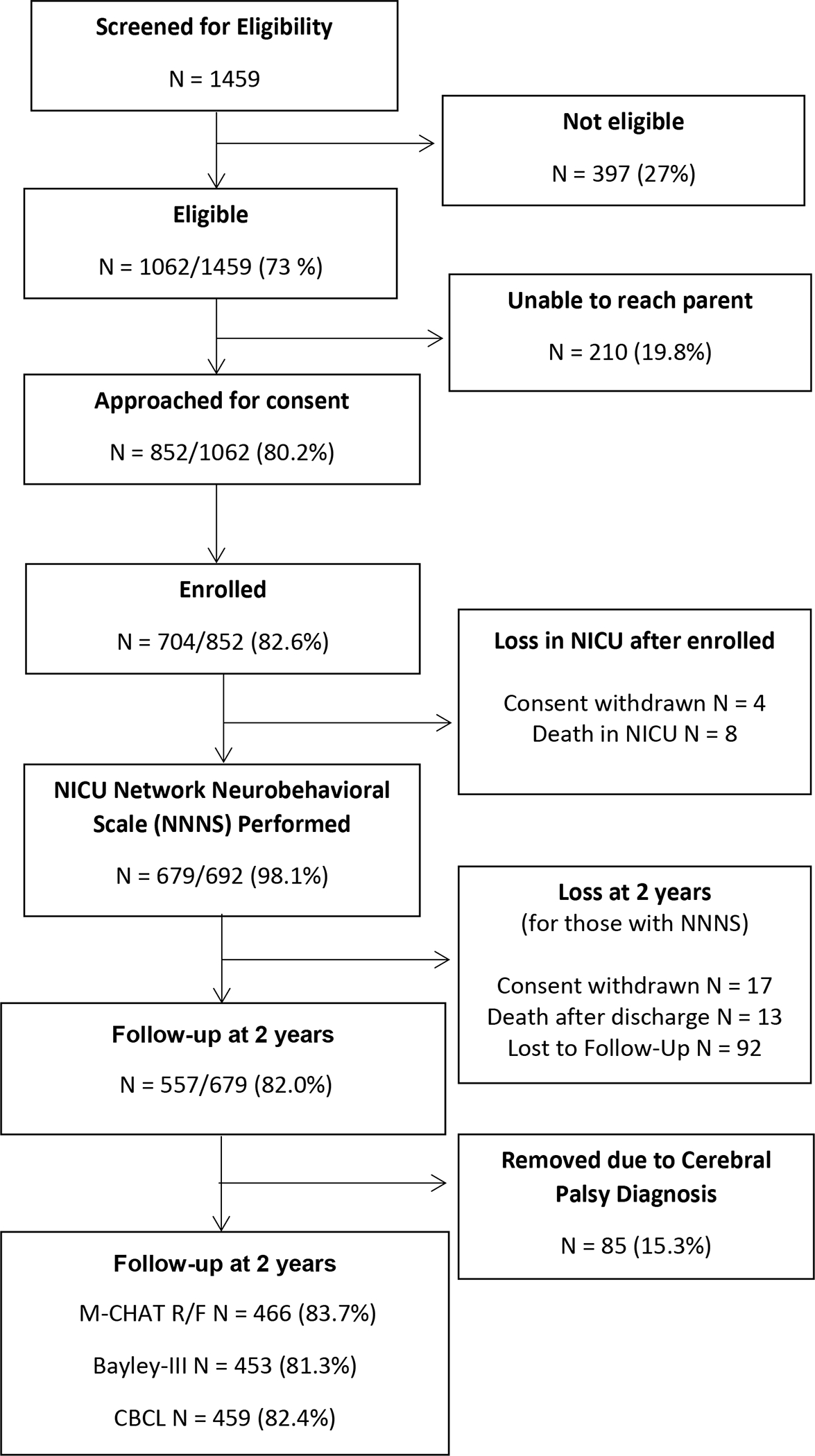
Participant Flowchart
At discharge from the NICU, 97 infants (21.1%) exhibited NNNS profile 5 and 29 infants (6.3%) had NNNS profile 6. Approximately 4.5% of the sample (n = 21) had both a positive M-CHAT-R/F screen and a predischarge NNNS profile 5 (n=19) or 6 (n=2). Two or more medical morbidities were identified in 78 infants (16.8%) during NICU stay. Table 2 displays demographic summaries for maternal and infant variables which were included in GEE models reported below.
Table 2.
Demographic Differences Between M-CHAT Screens
| M-CHAT Negative |
M-CHAT Positive |
p | |
|---|---|---|---|
| n = 415 (89.06%) | n = 51 (10.94%) | ||
| Maternal Demographics | |||
| Age – M (SD) | 29.27 (6.15) | 29.28 (7.50) | .991 |
| Education | |||
| < High schoola/General education diploma | 46 (10.04%) | 10 (2.18%) | .075 |
| >= High schoola/General education diploma | 362 (79.04%) | 40 (8.73%) | |
| Minority Race/Ethnicity | |||
| Yes | 224 (48.70%) | 36 (7.83%) | .032* |
| No | 185 (40.22%) | 15 (3.26%) | |
| Partner Status | |||
| Single | 83 (18.08%) | 32 (6.97%) | .006* |
| Has Partner | 325 (10.81%) | 19 (4.14%) | |
| Socioeconomic Status (SES) | |||
| Hollingshead Level 5 (low SES) | 32 (6.97%) | 4 (0.87%) | 1.00 |
| Hollingshead 1–4 | 376 (81.92%) | 47 (10.24%) | |
| Depression Diagnosisb | |||
| Yes | 82 (17.98%) | 17 (3.73%) | .033* |
| No | 323 (70.83%) | 34 (7.46%) | |
| Infant Demographics | |||
| Sex | |||
| Male | 214 (46.02%) | 30 (6.45%) | .336 |
| Female | 200 (43.01%) | 21 (4.52%) | |
| Birth weight (grams) – M (SD) | 970.96 (377.32) | 854.98 (259.74) | .005* |
| PMAc (weeks) - M (SD) | 27.17 (1.84) | 26.83 (2.17) | .278 |
| Intrauterine growth restrictiond | |||
| <10th Percentile | 32 (6.91%) | 9 (1.94%) | .019* |
| >= 10th Percentile | 380 (82.07%) | 42 (9.07%) | |
| Multiple Birth | |||
| Yes | 124 (26.67%) | 13 (2.80%) | .510 |
| No | 290 (62.37%) | 38 (8.17%) |
Note. M = mean, SD = standard deviation
= high school
= depression diagnosis includes whether mother self-reported prenatal depression and/or depression during pregnancy
= postmenstrual age
indicates statistical significance
Neonatal Factors and M-CHAT-R/F Screening
We ran GEE models with and without covariates. Covariates for these models were chosen based on statistical association with M-CHAT-R/F screening outcome. In unadjusted and adjusted GEE models, infants with 2–4 medical comorbidities and infants with high neurobehavioral risk (NNNS profiles 5 or 6) were significantly more likely to have an M-CHAT-R/F positive screens than infants with fewer medical comorbidities (Table 3). For infants with 2–4 comorbidities, the adjusted odds for having a positive M-CHAT-R/F screen were 2.65 (95% CI: 1.27, 5.54). For infants with high neurobehavioral risk (NNNS profiles 5 or 6), the adjusted odds of a positive M-CHAT-R/F were 2.00 (95% CI: 1.02, 3.94). When examining NNNS Profile 5 alone, the adjusted odds of a positive M-CHAT-R/F were 2.76 (95% CI: 1.38, 5.54). NNNS Profile 6 alone did not predict M-CHAT-R/F screen results. The interaction between the medical complications index (2–4 comorbidities) and high neurobehavioral risk did not significantly predict M-CHAT-R/F screen results. We included interaction terms for the medical complications index by both NNNS Profile 5 and 6, but neither interaction was a significant predictor of M-CHAT-R/F screen result.
Table 3.
Predicting M-CHAT Screening Status with Medical Complications Index and NNNS Profiles
| Predictors | N | B | SE | Sig | Unadjusted OR | Adjusted ORa | 95% CI (aORb) |
|---|---|---|---|---|---|---|---|
| Medical | |||||||
| 2–4 Comorbidities | 76 | .98 | .38 | .010* | 2.89 | 2.65 | 1.27, 5.54 |
| 0–1 Comorbidities (reference) | 379 | ||||||
| NNNS | |||||||
| Profiles 5–6 | 123 | .69 | .35 | .044* | 2.10 | 2.00 | 1.02, 3.94 |
| Profile 5 only | 95 | 1.02 | .36 | .004* | 2.56 | 2.76 | 1.38, 5.54 |
| Profile 6 only | 28 | −.81 | .82 | .322 | .78 | .446 | .09, 2.21 |
| Profiles 1–4 (reference) | 327 | ||||||
| Medical X NNNS Interaction | |||||||
| 2–4 by 5–6 | 26 | −.30 | .74 | .691 | .75 | .74 | .17, 3.19 |
| 2–4 by 5 | 17 | .02 | .81 | .985 | .95 | 1.02 | .21, 4.97 |
| 2–4 by 6 | 9 | −.18 | 1.58 | .908 | .76 | .83 | .04, 18.39 |
Note.
= adjusted for demographic variables including maternal education, partner status, race/ethnicity, SES, maternal depression, infant sex, birth weight, postmenstrual age
= adjusted odds ratio
= significant at p < .05
2-Year Outcomes
Table 4 shows correlations between the M-CHAT-R/F positive screens and Bayley-III scores and CBCL scores. Toddlers who screened positive on the M-CHAT-R/F had lower Bayley-III Cognitive, Language and Motor Composite scores than those who screened negative, with Cohen’s d effect sizes of .82–1.21. Compared with toddlers screening negative on the M-CHAT-R/F, toddlers who screened positive had significantly higher t-scores on the CBCL Internalizing and Externalizing Problem scales as well as Total problems with Cohen’s d effect sizes of −.84 to −.99. Children who screened positive on the M-CHAT-R/F also had significantly higher Withdrawn t-scores and PDD subscale t-scores compared with those who screened negative with Cohen’s d effect sizes of −1.22 to −1.42 (Table 4).
Table 4.
Bayley-III and CBCL outcomes between positive and negative M-CHAT R/F screens
| M- CHAT |
n | Mean | SD | t | df | p | Cohen’s d | |
|---|---|---|---|---|---|---|---|---|
| Bayley | ||||||||
| Cognition Composite | Positive | 49 | 83.67 | 15.64 | 5.43 | 451 | < .001 | 0.82 |
| Negative | 404 | 94.99 | 13.53 | |||||
| Motor Composite | Positive | 49 | 74.27 | 12.45 | 7.98 | 451 | < .001 | 1.21 |
| Negative | 404 | 91.73 | 14.96 | |||||
| Language Composite | Positive | 49 | 82.78 | 15.84 | 7.82 | 53.49 | < .001 | 1.18 |
| Negative | 404 | 96.26 | 10.74 | |||||
| CBCL | ||||||||
| Internalizing Problems | Positive | 50 | 55.68 | 11.82 | −6.19 | 457 | <.001 | −0.93 |
| Negative | 409 | 46.30 | 9.89 | |||||
| Externalizing Problems | Positive | 50 | 57.26 | 12.46 | −5.62 | 57.87 | < .001 | −0.84 |
| Negative | 409 | 48.21 | 10.52 | |||||
| Total Problems | Positive | 50 | 58.32 | 12.52 | −6.61 | 457 | < .001 | −0.99 |
| Negative | 409 | 47.96 | 10.19 | |||||
| Withdrawn Subscale | Positive | 50 | 62.10 | 10.89 | −5.59 | 51.90 | < .001 | −1.42 |
| Negative | 409 | 53.37 | 5.34 | |||||
| PDD Subscale | Positive | 50 | 62.12 | 10.77 | −5.23 | 52.78 | < .001 | −1.22 |
| Negative | 409 | 54.00 | 6.00 |
Note. CBCL = Child Behavior Checklist, clinical cut-off scores for Bayley composite scales < 85, clinical cut-off scores for CBCL t-scores ≥ 64, PDD = Pervasive Developmental Disorders.
DISCUSSION
Our findings showed that neonatal neurobehavior and medical morbidities were associated with positive M-CHAT-R/F screens at age 2 in toddlers born very preterm. Infants with hypo-aroused neurobehavior and those with a greater number of medical morbidities were more likely to screen positive on the M-CHAT-R/F. Additionally, the subgroup of infants with positive M-CHAT-R/F screens had lower Bayley composite scores and higher CBCL problem scores than infants with negative M-CHAT-R/F screen results. These developmental differences at age 2 were present after controlling for demographic factors, suggesting a subset of symptoms for which intervention may be effective. These findings highlight the connection between NICU complications and positive M-CHAT-R/F screens at 2-years of age, before the formal diagnosis of autism.
The current study adds to existing literature utilizing the NOVI cohort by expanding on the association between neonatal risk and developmental outcomes to include M-CHAT-R/F outcomes, with previous findings suggesting that infants with high neurobehavioral risk on the NNNS and the presence of medical morbidities were more likely to have poor Bayley-III and CBCL outcomes at 24 months(10). Taken together, this set of findings supports the idea the neonatal risk factors can be important indicators of future behavioral and developmental outcomes, including positive screens for autism, in high-risk infants born preterm.
The findings from the present study also align with previous work with infants born extremely preterm, finding that similar medical morbidities were also related to positive M-CHAT screens(20). Previous work showed that infants born at < 29 weeks of gestation without medical morbidities were more likely to score higher on all Bayley-III scales, with a positive Bayley-III outcome for this Canadian sample defined as a composite score ≥ 100 (21). Previous work has not, however, related neonatal neurobehavior to M-CHAT or M-CHAT-R/F screens in preterm populations. The finding of the hypo-aroused neurobehavior profile predicting positive M-CHAT-R/F screens contributes to a growing body of literature relating NNNS scores, including profiles, to developmental outcomes in preterm and other populations(7–9). Hypo-aroused neurobehavior in the NOVI sample is marked by increased lethargy, hypotonicity, non-optimal reflexes, poor quality of movement, poor self-regulation and low arousal. Some literature suggests that children with autism have lower baseline/resting heart rate variability compared with typically developing peers, suggesting dysregulation in the autonomic nervous system(22). This type of nervous system dysregulation could be associated with hypo-aroused neurobehavior in infants born preterm, but continued research is necessary to better understand the association between neonatal hypo-aroused neurobehavior and autism screening. In our study there were no significant interactions between NNNS profiles and medical morbidity scores suggesting that neurobehavior and medical comorbidities are independently associated with M-CHAT-R/F screens. Our findings suggest that the presence of medical comorbidities or atypical neonatal neurobehavior, specifically measured as NNNS hypo-aroused neurobehavior, might identify a subset of high-risk infants born very preterm at NICU discharge.
These findings take on added importance when we examine the developmental characteristics at 2-year follow-up of the children in our study with positive M-CHAT-R/F. These children had lower scores on Bayley-III Composite Cognitive, Language and Motor composites at two years adjusted age. Previous work examining the association between M-CHAT screening and Bayley-III scales in infants with extremely low gestational age also found that lower Bayley-III scores were associated with positive M-CHAT screens(5). However, our findings are noteworthy in that the scores on all three composites in the M-CHAT-R/F positive group were 1–2 SD below the standardized means in contrast to the scores in the M-CHAT-R/F negative group, which were within normal limits. The magnitude of these differences was also supported by the large effect sizes (Cohen’s d = 0.82 – 1.21).
Our results also show that children born very preterm who screened positive on the M-CHAT-R/F had more Internalizing and Externalizing problems on the CBCL than their negative screen counterparts. Children who screened positive also had higher scores on Withdrawn subscale and the PDD subscale, which are autism-related scales. Average withdrawn and PDD scores for children who screened M-CHAT-R/F positive were approaching the clinical cut-off of 64 and the effect sizes were large, suggesting that although specificity and sensitivity of the M-CHAT-R/F have been questioned, this measure may be useful as an early screener of autism-related symptoms in a very preterm sample. Previous work examining the use of CBCL with a sample of term children formally diagnosed with autism found their CBCL scores were significantly higher than the scores of typical peers(23). Furthermore, previous research with infants born preterm only found associations between M-CHAT positive screens and increased internalizing problems on the CBCL(12). The broad CBCL scale differences seen in the current study may reflect behavioral challenges in this sample born at < 30 weeks of gestation, where we found moderate to large effect sizes, although not as large as the effects seen with Bayley-III composites.
These differences in child development have the potential to be mitigated by targeted early intervention guided by identifying infants born preterm at NICU discharge using the NNNS exam and in the context of varied medical comorbidities. Although the NNNS is not a universally implemented measure of neonatal neurobehavior, the findings of our study coupled with previous work (showing that dysregulated NNNS profiles predict long-term outcomes) (10) support the use of NNNS as a predischarge screening measure in the NICU. Knowledge of infants’ NNNS profile results would enable pediatric care providers to provide closer monitoring for autism symptoms in children who were born very preterm with medical or neurobehavioral complications.
A limitation of this study is that we do not yet know which toddlers who screened positive on the M-CHAT-R/F will meet criteria for a formal diagnosis of autism. Regardless of this, children who screened positive on the M-CHAT-R/F had worse developmental and behavioral outcomes at age 2 compared with those who screened negative, suggesting that the M-CHAT-R/F may be identifying a subgroup of children with additional developmental challenges that may differ from those related to autism.
The use of the M-CHAT-R/F to identify children with these developmental and behavioral difficulties brings to question whether this measure may be a useful screening tool for global developmental delays, alongside other common developmental screeners, regardless of later autism diagnosis. The M-CHAT-R/F is low-cost, commonly administered, and may lead to early identification of developmental delays prior to administration of more expensive, time-consuming assessments. The findings from this study may assist in improving screening accuracy, improving interpretation of M-CHAT-R/F results, and to increase referral for services targeted toward infants born very preterm with medical comorbidities or high-risk neurobehavior. Continued research regarding utility of the M-CHAT-R/F may also assist in the creation of targeted interventions for infants born very preterm who experience neurobehavioral or medical conditions during NICU stay.
Funding/Support:
Supported by the National Institutes of Health (Award Number R01HD072267 to BL, MO); NICHD Neonatal Research Network (grants 2UG1HD027904 and 5UG1HD068284 to BL, CM). Disclaimer: The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations:
- NICU
Neonatal Intensive Care Unit
- NNNS
NICU Network Neurobehavioral Scales
- M-CHAT-R/F
Modified Checklist for Autism in Toddlers Revised with Follow-up
- CBCL
Child Behavior Checklist
- GEE
Generalized estimating equations
Footnotes
Conflict of Interest Disclosures: The authors have no conflicts of interest relevant to this article to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Crump C, Sundquist J, Sundquist K. Preterm or Early Term Birth and Risk of Autism. Pediatrics. 2021. Sep 1;148(3):e2020032300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van ‘t Hof M, Tisseur C, van Berckelear-Onnes I, van Nieuwenhuyzen A, Daniels AM, Deen M, et al. Age at autism spectrum disorder diagnosis: A systematic review and meta-analysis from 2012 to 2019. Autism. 2021. May;25(4):862–73. [DOI] [PubMed] [Google Scholar]
- 3.Kuban KCK, O’Shea TM, Allred EN, Tager-Flusberg H, Goldstein DJ, Leviton A. Positive Screening on the Modified Checklist for Autism in Toddlers (M-CHAT) in Extremely Low Gestational Age Newborns. J Pediatr. 2009. Apr;154(4):535–540.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robins DL, Casagrande K, Barton M, Chen CMA, Fein D. Validation of the Modified Checklist for Autism in Toddlers, Revised With Follow-up (M-CHAT-R/F). 2014;133(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Limperopoulos C, Bassan H, Sullivan NR, Soul JS, Robertson RL, Moore M, et al. Positive Screening for Autism in Ex-preterm Infants: Prevalence and Risk Factors. Pediatrics. 2008. Apr 1;121(4):758–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lester BM, Tronick EZ, in collaboration with T. Berry Brazelton MD. The Neonatal Intensive Care Unit Network Neurobehavioral Scale Procedures. Pediatrics. 2004. Mar 1;113(Supplement_2):641–67. [PubMed] [Google Scholar]
- 7.El-Dib M, Massaro AN, Glass P, Aly H. Neurobehavioral assessment as a predictor of neurodevelopmental outcome in preterm infants. J Perinatol. 2012. Apr;32(4):299–303. [DOI] [PubMed] [Google Scholar]
- 8.Bowers K, Khoury J, Sucharew H, Xu Y, Chen A, Lanphear B, et al. Early infant attention as a predictor of social and communicative behavior in childhood. Int J Behav Dev. 2019. May;43(3):204–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wouldes TA, Woodward LJ. Neurobehavior of newborn infants exposed prenatally to methadone and identification of a neurobehavioral profile linked to poorer neurodevelopmental outcomes at age 24 months. Jacobson S, editor. PLOS ONE. 2020. Oct 16;15(10):e0240905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGowan EC, Hofheimer JA, O’Shea TM, Kilbride H, Carter BS, Check J, et al. Analysis of Neonatal Neurobehavior and Developmental Outcomes Among Preterm Infants. JAMA Netw Open. 2022. Jul 18;5(7):e2222249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salley B, Sheinkopf SJ, Neal-Beevers AR, Tenenbaum EJ, Miller-Loncar CL, Tronick E, et al. Infants’ early visual attention and social engagement as developmental precursors to joint attention. Dev Psychol. 2016. Nov;52(11):1721–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bradshaw J, Klin A, Evans L, Klaiman C, Saulnier C, McCracken C. Development of attention from birth to 5 months in infants at risk for autism spectrum disorder. Dev Psychopathol. 2020. May;32(2):491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGowan EC, Hofheimer JA, O’Shea TM, Carter BS, Helderman J, Neal CR, et al. Sociodemographic and medical influences on neurobehavioral patterns in preterm infants: A multi-center study. Early Hum Dev. 2020. Mar;142:104954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bassler D, Stoll BJ, Schmidt B, Asztalos EV, Roberts RS, Robertson CMT, et al. Using a Count of Neonatal Morbidities to Predict Poor Outcome in Extremely Low Birth Weight Infants: Added Role of Neonatal Infection. Pediatrics. 2009. Jan 1;123(1):313–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bayley N Bayley Scales of Infant and Toddler Development. 3rd ed. Harcourt Assessment; 2006. [Google Scholar]
- 16.Achenbach T, Rescorla L. Manual for the ASEBA preschool forms & profiles. University of Vermont, Research Center for Children, Youth, & Families; 2000. [Google Scholar]
- 17.Nylund KL, Asparouhov T, Muthén BO. Deciding on the Number of Classes in Latent Class Analysis and Growth Mixture Modeling: A Monte Carlo Simulation Study. Struct Equ Model Multidiscip J. 2007. Oct 23;14(4):535–69. [Google Scholar]
- 18.Schmidt B, Asztalos EV, Roberts RS, Robertson CMT, Sauve RS, Whitfield< MF. Impact of Bronchopulmonary Dysplasia, Brain Injury, and Severe Retinopathy on the Outcome of Extremely Low-Birth-Weight Infants at 18 Months: Results From the Trial of Indomethacin Prophylaxis in Preterms: Obstet Gynecol Surv. 2003. Aug;58(8):524–5. [DOI] [PubMed] [Google Scholar]
- 19.Kim SH, Joseph RM, Frazier JA, O’Shea TM, Chawarska K, Allred EN, et al. Predictive Validity of the Modified Checklist for Autism in Toddlers (M-CHAT) Born Very Preterm. J Pediatr. 2016. Nov;178:101–107.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore T, Johnson S, Hennessy E, Marlow N. Screening for autism in extremely preterm infants: problems in interpretation: Autism Screening in Extremely Preterm Infants. Dev Med Child Neurol. 2012. Jun;54(6):514–20. [DOI] [PubMed] [Google Scholar]
- 21.Asztalos E, Church P, Riley P, Fajardo C, Shah P, for the Canadian Neonatal Network and Canadian Neonatal Follow-up Network Investigators. Neonatal Factors Associated with a Good Neurodevelopmental Outcome in Very Preterm Infants. Am J Perinatol. 2016. Sep 2;34(04):388–96. [DOI] [PubMed] [Google Scholar]
- 22.Lory C, Kadlaskar G, McNally Keehn R, Francis AL, Keehn B. Brief Report: Reduced Heart Rate Variability in Children with Autism Spectrum Disorder. J Autism Dev Disord. 2020. Nov;50(11):4183–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pandolfi V, Magyar CI, Dill CA. Confirmatory Factor Analysis of the Child Behavior Checklist 1.5–5 in a Sample of Children with Autism Spectrum Disorders. J Autism Dev Disord. 2009. Jul;39(7):986–95. [DOI] [PMC free article] [PubMed] [Google Scholar]