Fig. 6.
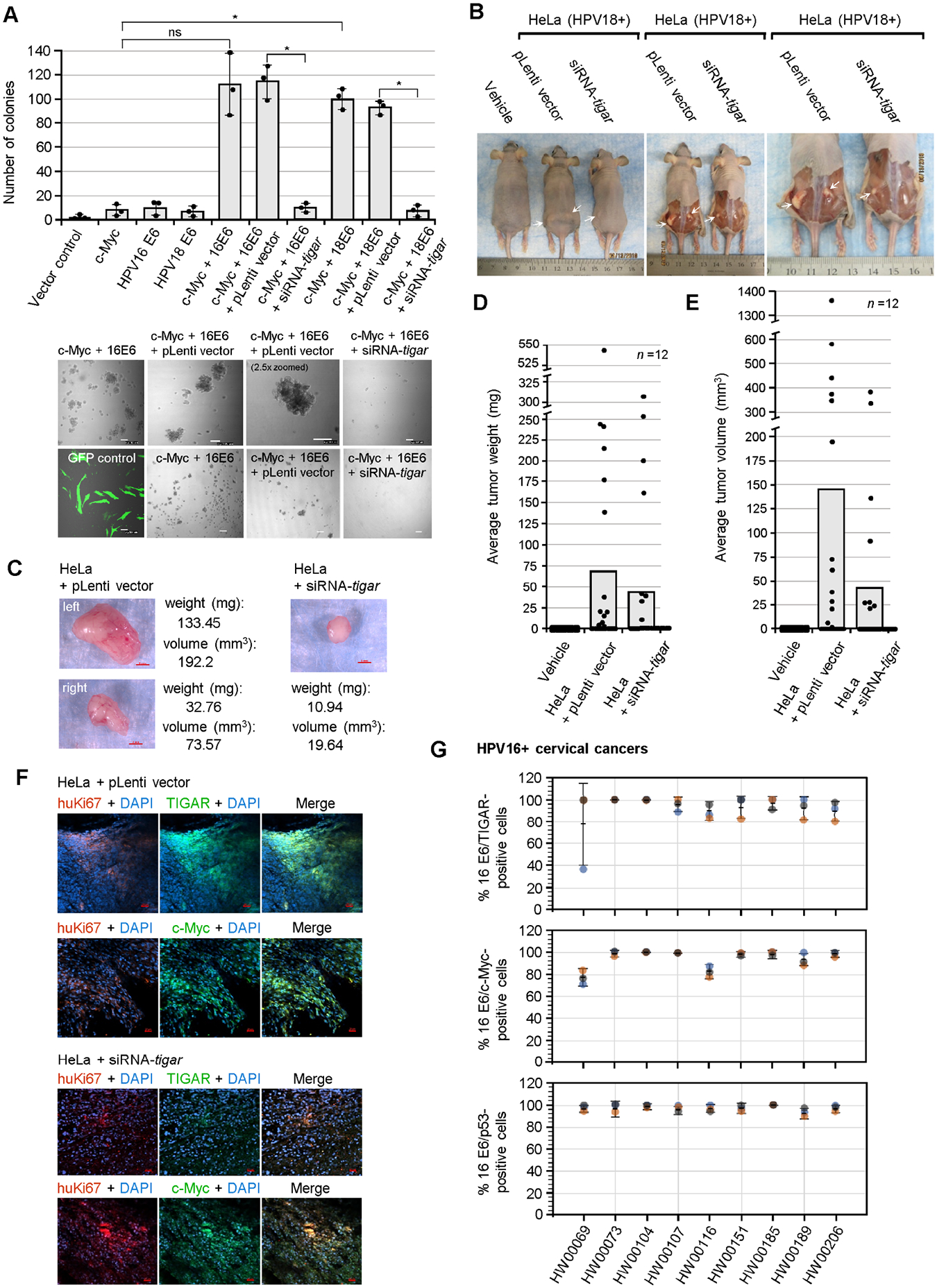
TIGAR is required for the oncogenic cooperation between E6/c-Myc and hrHPV-induced tumorigenesis in vivo. (A) The role of TIGAR in oncogenic cellular transformation by the viral E6 oncoprotein and c-Myc was assessed by cotransfecting primary human dermal fibroblasts (neonatal foreskin, HDFn; ATCC) with various expression constructs for HPV16/18 E6, c-Myc, or an empty CβS vector and then transducing the cells with lentiviral-siRNA-tigar or the pLenti vector as a negative control. The transfection efficiency was evaluated using a N3-pEGFP plasmid and performing fluorescence-microscopy with a DIC filter overlay to visualize GFP expression (bottom left). Soft agar colony assays were performed over a period of 3 weeks and the transformed anchorage-independent spheroids were quantified by counting. Representative micrographs of the transformed colonies are shown in the lower panels. Scale bar, 100 μm. Data is mean ± SD. N-value = 3. The asterisks denote statistical significance as determined using unpaired two-tailed Student’s t-tests (*P<0.0332, not significant (ns) 0.1234). (B) To determine if TIGAR functions are required for hrHPV-induced tumorigenesis in vivo, athymic NIH III-nude mice were subcutaneously engrafted over each hind flank with HPV18+ HeLa cells that were transduced with lentiviral-siRNA-tigar to inhibit TIGAR expression, or an empty lentiviral (pLenti) vector as negative control. Alternatively, a group of animals was injected with the vehicle alone (i.e., sterile Ca2+/Mg2+-free PBS, pH 7.4) for comparison (n-value = 12). After 8 weeks, the experimental animals were humanely sacrificed and necropsies were performed to analyze the growth of the primary xenograft tumors (a Kaplan-Meier plot of tumor development, as well as graphs of the relative percentages of animals that developed tumors or exhibited bilateral tumor growth are provided in Figs. S9A–C). The arrows in B indicate the significantly larger tumor masses present in the HeLa/pLenti vector animals as compared to the HeLa/lentiviral-siRNA-tigar animals. (C) The harvested tumor masses were weighed and their volumes were determined by measuring with a digital caliper. Representative images are provided. Scale bar, 2 mm. (D) The tumors derived from HeLa/lentiviral-siRNA-tigar engrafted animals exhibited a reduced average weight as compared to the HeLa/pLenti vector animals. (E) The tumor masses from the HeLa/lentiviralsiRNA-tigar animals also exhibited a smaller average volume than the HeLa/pLenti vector sample group. (F) The expression of TIGAR and c-Myc (green signals) in the xenograft tumor tissues from engrafted HeLa/pLenti vector and HeLa/siRNA-tigar experimental animals was analyzed by immunofluorescence-confocal microscopy. The engrafted HeLa tumor cells were visualized by co-staining the samples with an Anti-human-Ki67 (huKi67) primary antibody (red signal). DAPI nuclear-staining (blue signal) is included in the merged images for reference. Scale bar, 20 μm. (G) The TIGAR, c-Myc, and p53 proteins are detectably expressed in the E6-positive tumor cells of primary HPV16+ cervical cancer patient isolates. Clinically biopsied HPV16+ cervical cancer tissue specimens were provided by the Pathology Shared Resource of the University Hawaii Cancer Center and the relative percentages of HPV16 E6/TIGAR-positive, HPV16 E6/c-Myc-positive, and HPV16 E6/p53-positive cells per field were quantified by immunofluorescence-confocal microscopy and counting triplicate visual fields at 200x magnification (representative micrographs for the HPV16+ clinical samples as well as the HPV-negative HFL1 antibody control are provided in Figs. S10A–B). The error bars are mean ±SD, n-value = 3.
