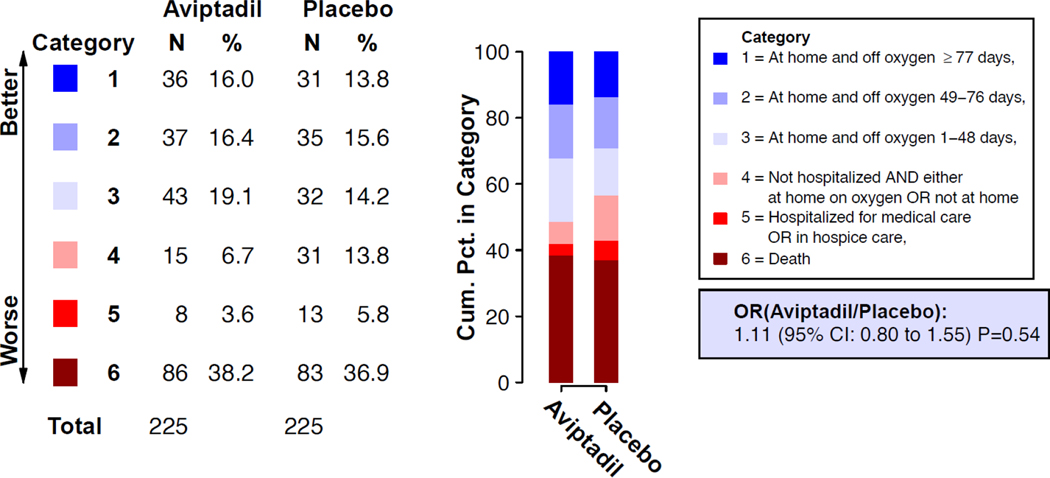
Figure 1.
Primary 6-category ordinal outcome at Day 90 for the aviptadil versus placebo comparison. Category percentages at Day 90 for the aviptadil group and the placebo group are shown. The summary odds ratio (OR) was estimated with the use of a proportional odds model that was stratified by disease severity at entry. An OR > 1.0 favors aviptadil. Estimated for the 450 participants with known status on Day 90. See Supplementary Appendix Figure S4 for related figure that includes imputation for those with unknown Day 90 status.