Figure 2. Project MEDIUM Study Design.
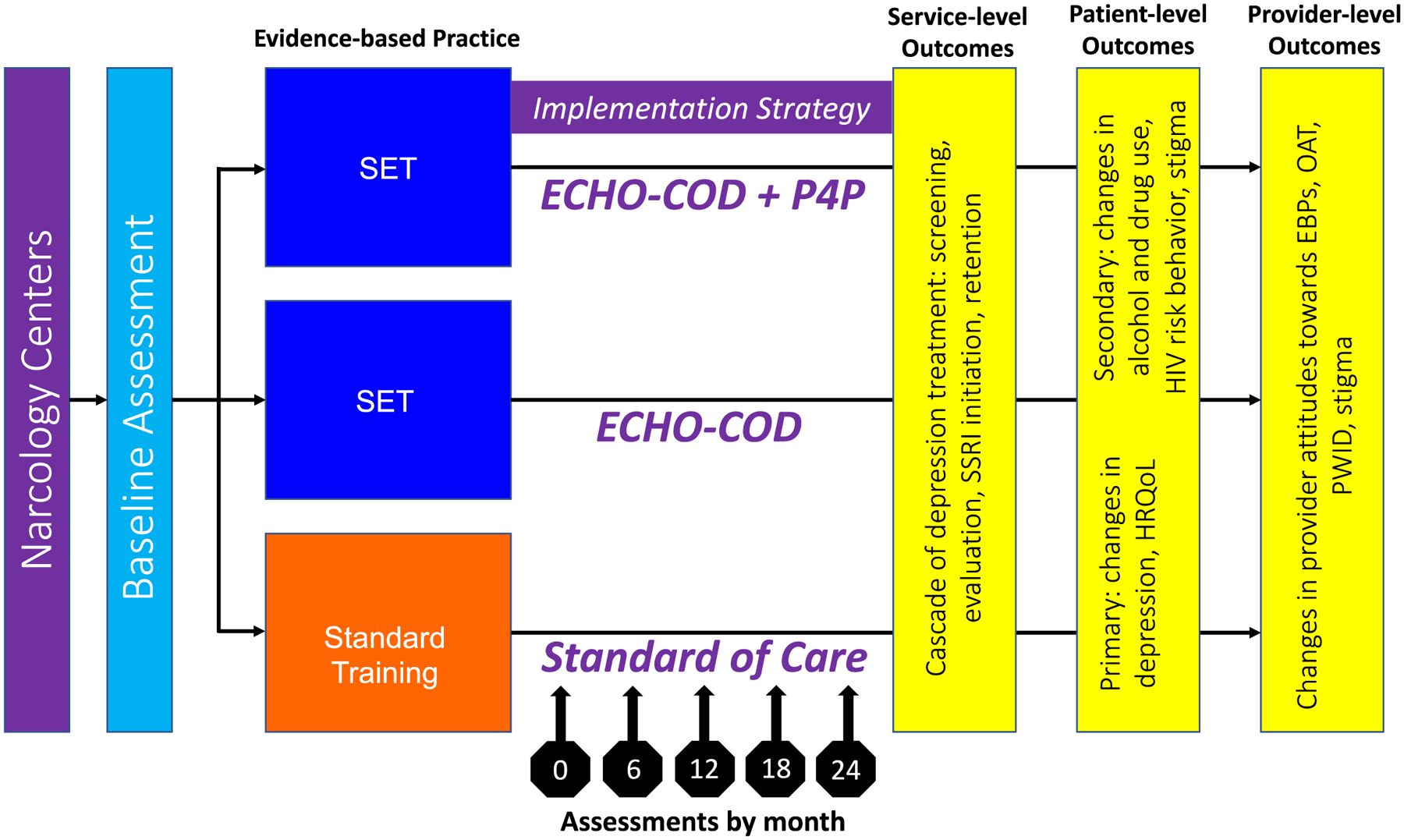
The MEDIUM study had three arms: Standard of Care, ECHO-COD, and ECHO-COD+P4P. Assessments were carried out on baseline and every 6 months for 24 months. Service-, patient- and provider-level outcomes are presented.
Abbreviations: SET: Screening Evaluation Treatment; ECHO-COD: Extension for Community Healthcare Outcomes® modified for Co-occurring Disorders; P4P: Pay for Performance; HRQoL Health-related Quality of Life, EBP Evidence based practice, OAT opioid agonist therapies, PWID people who inject drugs.
