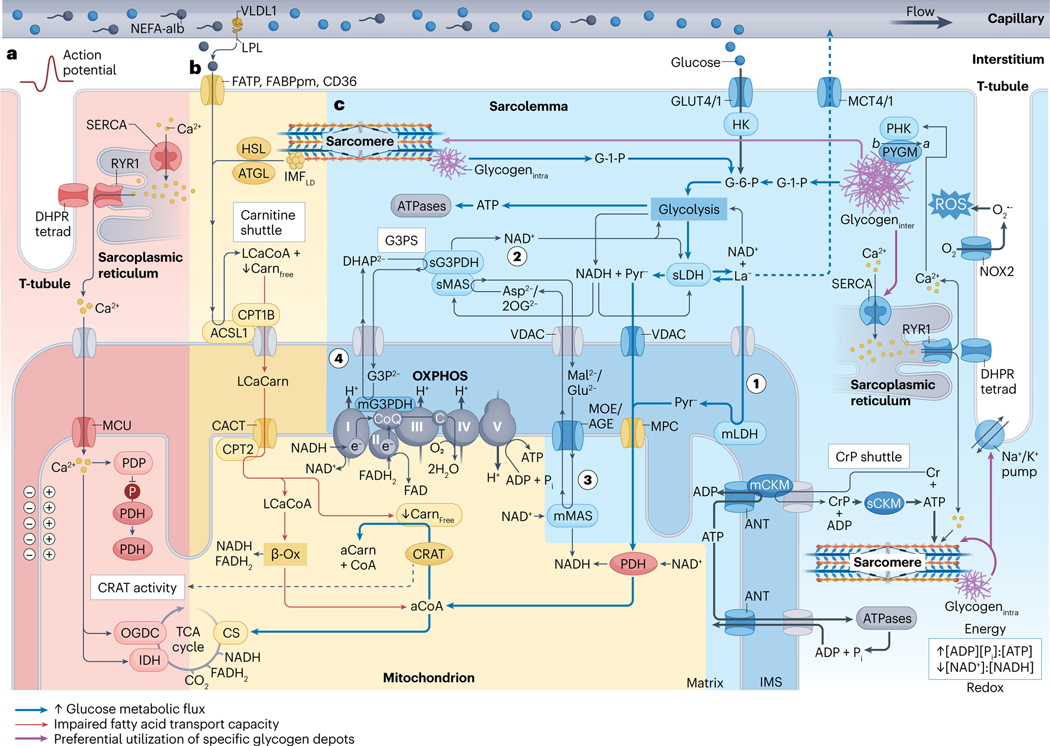
Fig. 2 |. Skeletal muscle metabolism during higher-intensity exercise.
a, Exercise-onset metabolic inertia (red area). Acetyl-carnitine (aCarn) abundance83–85 and the acetyl coenzyme A (aCoA)-producing capacities of carnitine acetyltransferase (CRAT)83,84 and pyruvate dehydrogenase (PDH)85 appear rate-limiting for oxidative adenosine triphosphate (ATP) regeneration at the onset of moderate-high intensity exercise. Contraction-induced calcium () transients promote mitochondrial uptake into the matrix space269 through the inner-membrane mitochondrial calcium uniporter (MCU) complex270,271. Increased matrix can upregulate PDH272 through activation of its phosphatase (PDP)273, and can upregulate isocitrate dehydrogenase (IDH)274 and 2-oxoglutarate dehydrogenase (OGDC)272,274,275 directly to fine-tune oxidative metabolism via stimulation of the tricarboxylic acid (TCA) cycle82. kinetics probably precede an allosteric86 rise in the [ADP][] to [ATP] and [creatine][] to [creatine phosphate] ratios in part because ADP and creatine (Cr) are buffered by the adenylate kinase (not shown) and creatine phosphate (CrP) shuttle reactions. Thus, synchrony between mechanisms of substrate provision, -feedforward and metabolite feedback regulation might underlie acute metabolic inertia. This could be particularly prominent in type II fibres, which have lower CRAT83 and MCU abundance21 and slower mitochondrial import rates276. Furthermore, metabolic inertia is more pronounced in metabolically compromised and older untrained adults, related to the lower CRAT activity and acetyl-carnitine content of muscle in these individuals84. b, Carbohydrates outcompete non-esterified fatty acids (NEFAs) for oxidation at higher intensities (yellow area). Muscle glucose uptake88 and carbohydrate utilization71,72,120 increases with exercise intensity. At workloads above maximum fat oxidation (>60–65% of maximal oxygen consumption ()) flux of pyruvate to acetyl-CoA progressively exceeds rates of TCA cycle entry at citrate synthase (CS), leading to depletion of the muscle free-carnitine (Carnfree) pool through CRAT-dependent acetylation to acetyl-carnitine83. After higher-intensity submaximal exercise, acetylation of the free-carnitine pool is greatest in type I fibres142. Insufficient free-carnitine availability would inhibit NEFA mitochondrial import at the first step of the carnitine shuttle — that is, carnitine palmitoyl transferase 1B (CPT1B) conjugation of carnitine to long-chain acyl-CoA (LCaCoA). Reduced fat oxidation is associated with diminished free-carnitine levels at ~70% of (ref. 72), whereas medium-chain NEFA metabolism bypasses carnitine shuttling and is maintained at higher submaximal workloads277. Therefore, free-carnitine levels appear rate-limiting for long-chain NEFA utilization at increasing exercise intensities. c, Lactate and pyruvate oxidation and NADH shuttles (blue area). Downstream of glycolysis, pyruvate (Pyr−) and/or lactate (La−) pass through voltage-dependent anion channels (VDAC), where lactate is converted to pyruvate by mitochondrial lactate dehydrogenase (mLDH) in the intermembrane space119 (step 1). Pyruvate then enters the mitochondrial matrix through the mitochondrial pyruvate carrier (MPC)81,119. The glycerol-3-phosphate (G3P2−) shuttle (G3PS) and malate (Mal2−)/aspartate (Asp2−) shuttle enables mitochondrial oxidation of lactate and pyruvate through compartmentalized redox shuttling. G3PS and MAS recycle extra-matrix nicotinamide adenine dinucleotide (NAD+) (step 2) and transport reducing power from glycolysis to the mitochondrial matrix. This occurs through reactions associated with Mal2− and Asp2− delivery into the matrix space81,119 (step 3) and G3P2− donation of electrons directly to coenzyme Q (CoQ) of the electron transport chain119 (step 4). As such, saturation of these shuttles increases lactate accumulation and upregulates the lactate-favouring LDHA isoform in vitro278. See Box 3 for discussion of CrP, intermyofibrillar and intramyofibrillar glycogen (glycogeninter and glycogenintra, respectively) and intermyofibrillar lipid droplet (IMFLD) stores, and section ‘Acute exercise muscle metabolism’ for details of their metabolism during acute exercise. -Ox, -oxidation; ACSL1, acyl-CoA synthetase long-chain family member 1; AGE, aspartate/glutamate exchanger; ANT, adenine nucleotide translocator; ATGL, adipose triacylglyceride lipase; denotes posttranslational modification of PYGM from its less-active form to the constitutively active form by PHK; C, cytochrome ; CACT, carnitine acylcarnitine translocase; mCKM mitochondrial creatine kinase muscle; sCKM, sarcoplasmic CKM; DHAP2−, dihydroxyacetone phosphate; DHPR tetrad, four dihydropyridine receptors associated with one ryanodine receptor 1 (RYR1); FABPpm, fatty acid binding protein plasma membrane; FAD, flavin AD; FADH2, reduced FAD; FATP, FA transporter protein; GLUT4/1; glucose transporters 4 and 1; G-1-P, glucose-1-phosphate; G-6-P, glucose-6-phosphate; HK, hexokinase; HSL, hormone-sensitive lipase; IMS, intermembrane space; LCaCarn, long-chain acyl carnitine; LPL, lipoprotein lipase; sLDH, sarcoplasmic LDH; mMAS, mitochondrial MAS; MCT4/1, monocarboxylate transporters 4 and 1; mG3PDH, mitochondrial glycerol-3-phosphate dehydrogenase; MOE, malate/2-oxoglutarate exchanger; NADH, reduced NAD; NEFA-alb, albumin-bound NEFA; NOX2, NAD phosphate oxidase 2; superoxide; 2OG2−, 2-oxoglutarate; OXPHOS, oxidative phosphorylation and associated respiratory complexes; PHK, muscle phosphorylase kinase; PYGM, glycogen phosphorylase muscle-associated; ROS, reactive oxygen species; SERCA, sarcoplasmic/endoplasmic reticulum -ATPase; sG3PDH, sarcoplasmic glycerol-3-phosphate dehydrogenase; sMAS, sarcoplasmic MAS; T-tubule, transverse tubule; VLDL1, very low density lipoprotein 1.