Figure 4. Chemical probes to study oxidative cysteine and methionine modification in thrombotic disorders.
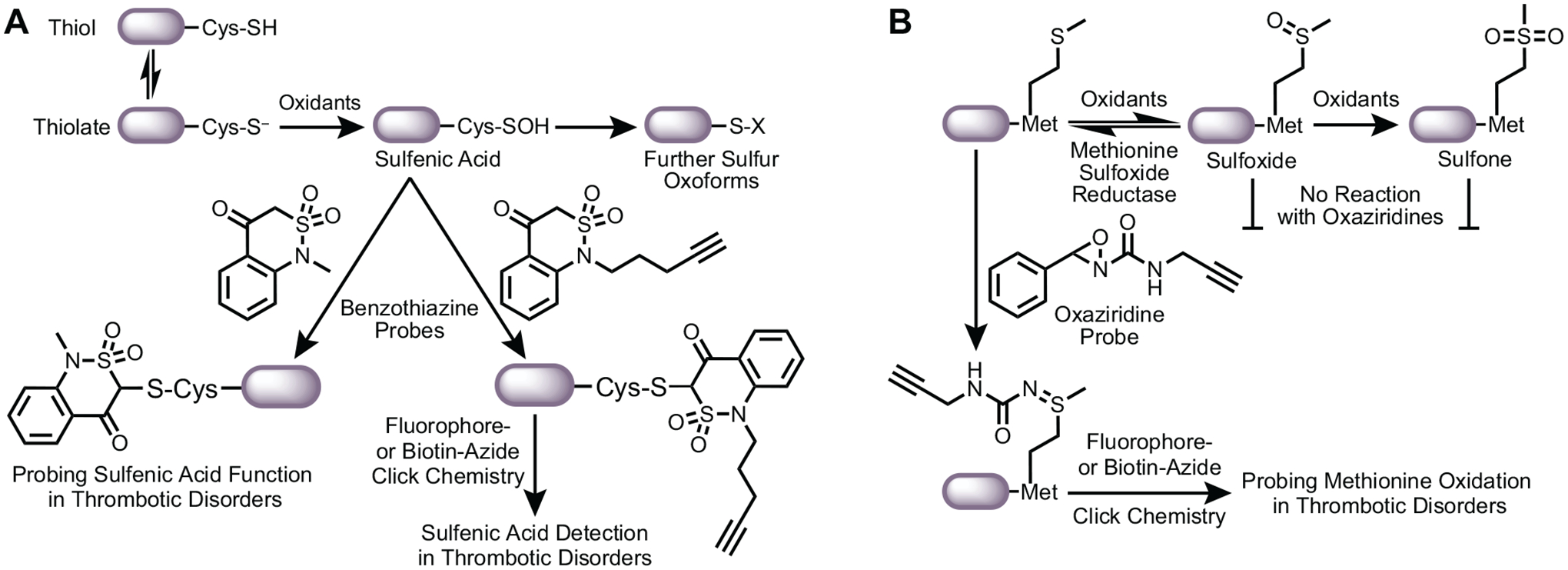
(A) Using carbon nucleophiles to probe for sulfenic acid detection and function in thrombosis. The cysteine thiol is in equilibrium with its deprotonated thiolate anion. The nucleophilic thiolate anion is then oxidized to a sulfenic acid by oxidants generated during oxidative stress. Sulfenic acid is both a nucleophile and an electrophile and is a precursor to further sulfur oxoforms (e.g. sulfinic and sulfonic acids). Sulfenic acids can also be converted to disulfides in the presence of proximal thiols. Carbon nucleophiles, such as benzothiazine-based probes developed by the Carroll laboratory, covalently and selectively target sulfenic acids. Benzothiazine (BTD) with an alkyne arm detects cysteine sulfenylation on proteins using click chemistry with azides in the context of thrombotic disorders. In addition, an alkyneless BTD can probe the function of the sulfenic acid as alkylation prevents further oxidation and reduction of the cysteine. Additional studies are essential to determine what proteins are sulfenylated and the impacts on protein function and subsequent thrombotic activity. (B) Methionine residues can be oxidized to methionine sulfoxides. This oxidative event is reversible with the enzyme methionine sulfoxide reductase. Further oxidation of the sulfoxide yields methionine sulfone, thought to be irreversible. Recent oxaziridine-based probes can label unoxidized methionines. Labeling of the methionine via a sulfimide linkage can be coupled with click chemistry to identify proteins that undergo oxidative methionine modification, as shown by a loss of probe labeling.
