Abstract
Background.
Community-acquired pneumonia (CAP) is a leading cause of hospital admissions and antimicrobial use. Clinical practice guidelines recommend switching from intravenous (IV) to oral antibiotics once patients are clinically stable.
Methods.
We conducted a retrospective cohort study of adults admitted with CAP and initially treated with IV antibiotics at 642 US hospitals from 2010 through 2015. Switching was defined as discontinuation of IV and initiation of oral antibiotics without interrupting therapy. Patients switched by hospital day 3 were considered early switchers. We compared length of stay (LOS), in-hospital 14-day mortality, late deterioration (intensive care unit [ICU] transfer), and hospital costs between early switchers and others, controlling for hospital characteristics, patient demographics, comorbidities, initial treatments, and predicted mortality.
Results.
Of 378 041 CAP patients, 21 784 (6%) were switched early, most frequently to fluoroquinolones. Patients switched early had fewer days on IV antibiotics, shorter duration of inpatient antibiotic treatment, shorter LOS, and lower hospitalization costs, but no significant excesses in 14-day in-hospital mortality or late ICU admission. Patients at a higher mortality risk were less likely to be switched. However, even in hospitals with relatively high switch rates, <15% of very low–risk patients were switched early.
Conclusions.
Although early switching was not associated with worse outcomes and was associated with shorter LOS and fewer days on antibiotics, it occurred infrequently. Even in hospitals with high switch rates, <15% of very low–risk patients were switched early. Our findings suggest that many more patients could be switched early without compromising outcomes.
Keywords: community-acquired pneumonia, switch therapy, antimicrobial stewardship, IV to oral
Pneumonia is a leading cause of hospital admissions and antibiotic use in the United States [1, 2]. Optimizing antibiotic treatment is important, as prolonged exposure can lead to increased drug resistance, toxicity, and Clostridium difficile infection [3, 4]. Most patients hospitalized with severe CAP are treated initially with intravenous (IV) antibiotics [5]. Switching to oral treatment as soon as feasible has many advantages, including reduced risk of infection, decreased hospital length of stay (LOS), and lower associated costs [6, 7]. Randomized trials have shown that switching to oral antibiotics is safe for most patients hospitalized with CAP [8-10]. For these reasons, the 2007 American Thoracic Society and Infectious Diseases Society of America (IDSA) guidelines and the 2016 IDSA Antimicrobial Stewardship guidelines strongly recommend a timely transition from IV to oral antibiotics as soon as patients are clinically stable [11, 12]. However, clinicians may hesitate to switch either because they fear oral antibiotics will not be effective or because there is no oral equivalent for the IV antibiotic that the patient is receiving [13, 14]. Unfortunately, the circumstances under which clinicians make this switch remain poorly understood. No large-scale studies have specifically evaluated switching practices across a representative sample of US hospitals. These practices (at the hospital or the clinician level) should be better understood both to validate the guideline recommendations and provide evidence to undergird quality improvement efforts.
We analyzed data from 642 US acute care hospitals to describe current IV to oral practice and associations between early switch therapy and clinical outcomes to determine whether early switching is associated with worse outcomes.
METHODS
Data Source
We used the Premier Healthcare Database (PHD; Premier Inc, Charlotte, NC), a large, all-payer inpatient hospital discharge database with information from more than 600 US hospitals. The participating hospitals provide detailed administrative, healthcare utilization, and financial data. The PHD includes patient sociodemographic information; discharge diagnostic codes (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9 CM]); and discharge status and date-stamped charge codes of all procedures, medications, and laboratory tests. The hospitals represent >20% of all US acute care hospital discharges, although urban and southern hospitals are overrepresented. Approximately 75% of contributing hospitals provide actual cost data; the rest provide charges and cost-to-charge ratios. As all PHD data are de-identified, the Cleveland Clinic Institutional Review Board deemed the study exempt.
Study Cohort
We included adult patients (aged ≥18 years) who were discharged between July 2010 and June 2015 from a participating hospital with a principal diagnosis of pneumonia or a principal diagnosis of sepsis or respiratory failure with a secondary diagnosis of pneumonia. To improve the specificity of pneumonia diagnoses, we also required that patients had a chest X ray or computed tomography scan and received antimicrobial therapy for at least 3 hospital days beginning in the emergency department or on hospital day 1. We included only 1 randomly selected hospitalization for patients with multiple qualifying admissions. We excluded patients with cystic fibrosis or lung transplantation (because they may not have oral antimicrobial options based on the epidemiology of infections in these populations) and those transferred from another hospital (since we could not assess their initial severities and outcomes). Because we were assessing antibiotic utilization, we excluded patients with secondary diagnosis codes for other infections (eg, cellulitis, endocarditis), patients with chronic ventilator dependence, and patients with positive urine culture within the first 3 hospital days. Patients with hospital-acquired pneumonia or ventilator-associated pneumonia were excluded. We also excluded patients who presumably could not receive oral medication on hospital day 3 (no charge codes for oral medications of any kind on hospital day 3), patients who were in the intensive care unit (ICU; hemodynamically unstable) on hospital day 3, and small fractions of patients missing data on any of discharge status, sex, point of origin, or costs.
We defined switching as discontinuation of IV antibiotics and initiation of oral antibiotics for the first time during the hospitalization and on the same day (no delay between the switch based on conventional dosing intervals). Patients whose IV to oral switch occurred by hospital day 3 were identified as early switchers. We chose day 3 as most CAP inpatients are switched after 2–4 days of IV therapy [15]. Among patients who were switched, we descriptively summarized the combinations of the initial IV and the oral antibiotic to which the patients switched. The relationships of switching to outcomes were analyzed at 2 levels to overcome potential confounding at the patient level and to evaluate correlates of hospital switch therapy practices. We compared patients switched on or before hospital day 3 (early switchers) with patients with similar characteristics who were switched later or not switched. We excluded patients who died or were discharged on or before hospital day 3 and those who stopped CAP antibiotics before hospital day 3 with no further antibiotics. Because sicker patients might be expected to continue IV antibiotics longer, leading to confounding by indication, we controlled analyses for initial antibiotic in the emergency department or on hospital day 1, which is associated with severity of illness. For outcomes analyses, we used the entire cohort of patients and the subgroup treated with only a third-generation cephalosporin + macrolide or with fluoroquinolone monotherapy for the first 3 days. We were particularly interested in this subgroup because these antibiotic regimens are the most commonly administered empiric therapies for inpatient CAP and because patients treated with them tend to have low mortality. We restricted hospital-level analyses to those with at least 100 otherwise eligible patients to have reasonably stable estimates of hospital early switch rates.
Baseline Variables
For each patient, we extracted demographics (age, sex, race, and insurance status), admission source, hospital size (number of beds), teaching status, US Census Bureau geographic region, and urban vs rural setting. Comorbidities were categorized using software provided by the Agency for Healthcare Research and Quality (Healthcare Cost and Utilization Project Comorbidity Software, version 3.1) based on the Elixhauser method as described previously [16]. Combined comorbidity scores were also calculated using the method described by Gagne et al [17]. Organ failure scores were derived from discharge diagnosis codes representing respiratory, cardiovascular, renal, hepatic, metabolic, and neurologic failure [18]. Initial CAP disease severity was assessed via indirect markers present on hospital day 1 (ie, admission to an ICU, invasive mechanical ventilation [IMV], and use of vasopressors). To account for disease severity on hospital day 3, we calculated the predicted mortality risk for each patient, using a previously validated model whose performance is similar to that of the Pneumonia Severity Index [19].
Outcomes
The main outcomes of interest were hospital LOS, 14-day in-hospital case fatality (day 3 through day 14), and the entire cost of hospitalization (eg, bed charge, medications, and laboratory tests). Patients discharged alive were considered to be alive at 14 days. Other outcomes of interest included indicators of clinical deterioration: transfer to an ICU, initiation of IMV, and vasopressor use after hospital day 3 (in contrast to measures of initial severity, which were limited to hospital day 1); total antibiotic days; and Clostridium difficile infection (CDI), defined by a new ICD-9 code (not present on admission) and/or positive laboratory test for C. difficile. Hospital readmission data were not available.
Statistical Analyses
Categorical variables were summarized by frequencies and proportions. Continuous variables were summarized as mean and standard deviation or median and quartiles (25th–75th percentiles) depending on distributional symmetry. To show patterns of antibiotic changes among switched patients, we classified the initial (IV) and subsequent (oral) antibiotics into classes, examined their marginal and joint distributions and, for each of the 6 most common initial IV categories, displayed the frequencies of the dozen most common successor oral antibiotic categories to which they were switched in a grouped bar chart. The relationships of switching to outcomes were analyzed at 2 levels: patient level and hospital level. A detailed description of the statistical methods is provided in Supplementary Material 1A. Outcome analyses were done using SAS 9.4 (SAS Institute Inc, Cary, NC). All tests were 2-sided, and P < .05 was considered statistically significant.
RESULTS
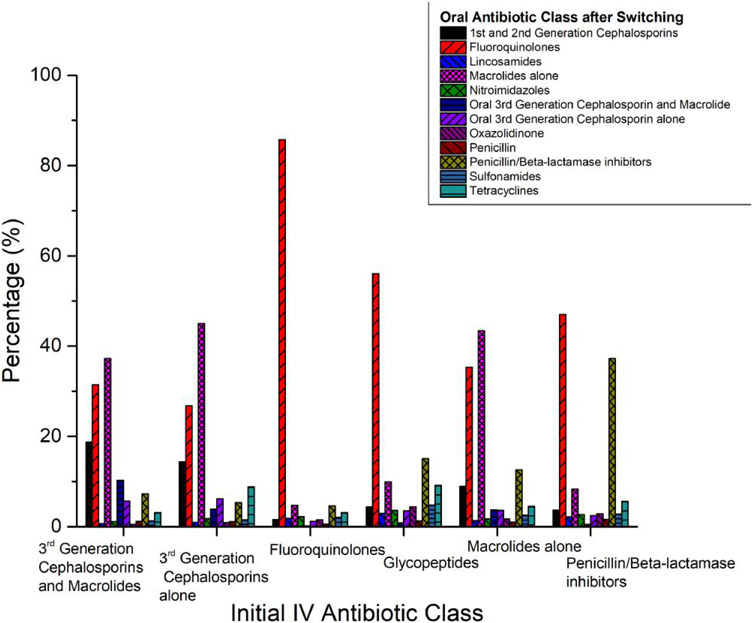
After applying the exclusion criteria, our sample consisted of 378 041 patients from 642 hospitals (Figure 1) who were treated with IV antibiotics on hospital day 1. Among these, 116 118 (30.7%) were switched to oral antibiotics before discharge, with 21 784 (5.8%) switched early (by day 3). Among the patients who were switched, most were on IV fluoroquinolones (28%), third-generation cephalosporins alone (23.1%), penicillin/beta-lactamase inhibitors (15.1%), third-generation cephalosporins + macrolide (11.8%), glycopeptides (combination or alone) (13.7%), and macrolides alone (4.6%) the day prior to switching. Patients were most frequently switched to fluoroquinolones (44.4%), macrolides alone (20.0%), and penicillin/beta-lactamase inhibitors (9.9%; Figure 2). Supplementary Table 1 shows the clinical and demographic characteristics of all early switchers vs others, and Table 1 restricts this comparison to the subgroup of patients initially treated with IV third-generation cephalosporin + macrolide or IV quinolone monotherapy. In this subgroup, patients who were switched early had comorbidity scores that were similar to those of other patients. Early switchers were more likely to have a principal diagnosis of respiratory failure, chronic pulmonary disease, been admitted to a healthcare facility in the previous 6 months, been admitted to the ICU, and be at large and urban hospitals. In this subgroup, 100% of those who switched early started on fluoroquinolone monotherapy, and none of the patients started on IV third-generation cephalosporin + macrolide were switched early. Supplementary Table 2 compares patients who were switched at any time to patients who were not switched during their hospital stay.
Figure 1.
Cohort inclusion-exclusion criteria for selection of hospitalizations. Abbreviations: CT, computed tomography; IMV, invasive mechanical ventilation; IV, intravenous; S. aureus, Staphylococcus aureus.
Figure 2.
Comparative distributions of oral antibiotic class after switching (colored bars, percentages of patients) by class of initial IV antibiotic (horizontal axis label). Abbreviation: IV, intravenous.
Table 1.
Comparison of the Clinical and Demographic Characteristics of Early Switchers vs Others Among Patients Initially Treated With Intravenous (IV) Third-Generation Cephalosporin Plus a Macrolide or IV Quinolone Monotherapy
| Factor | Total (N = 96 539) |
Early Switchers (N = 5606) |
Others (N = 90 933) |
P Value |
|---|---|---|---|---|
| Demographics | ||||
| Age, mean ± SD, y | 70.3 ± 15.7 | 68.7 ± 15.3 | 70.4 ± 15.7 | <.001† |
| Age group, no. (%), y | … | … | … | <.001§ |
| 18–49 | 10 683 (11.1) | 647 (11.5) | 10 036 (11.0) | … |
| 50–64 | 20 815 (21.6) | 1444 (25.8) | 19 371 (21.3) | … |
| 65–79 | 30 821 (31.9) | 1873 (33.4) | 28 948 (31.8) | … |
| ≥80 | 34 220 (35.4) | 1642 (29.3) | 32 578 (35.8) | … |
| Male, no. (%) | 43 119 (44.7) | 2326 (41.5) | 40 793 (44.9) | <.001§ |
| Female, no. (%) | 53 420 (55.3) | 3280 (58.5) | 50 140 (55.1) | <.001§ |
| Race, no. (%) | … | … | … | <.001§ |
| White | 73 949 (76.6) | 4211 (75.1) | 69 738 (76.7) | … |
| Black | 9474 (9.8) | 686 (12.2) | 8788 (9.7) | … |
| Hispanic | 857 (0.89) | 38 (0.68) | 819 (0.90) | … |
| Other | 12 207 (12.6) | 669 (11.9) | 11 538 (12.7) | … |
| Unknown | 52 (0.05) | 2 (0.04) | 50 (0.05) | … |
| Insurance status, no. (%) | … | … | … | <.001§ |
| Medicare | 68 333 (70.8) | 3856 (68.8) | 64 477 (70.9) | … |
| Medicaid | 7502 (7.8) | 511 (9.1) | 6991 (7.7) | … |
| Managed care | 11 616 (12.0) | 672 (12.0) | 10 944 (12.0) | … |
| Commercial indemnity | 2790 (2.9) | 145 (2.6) | 2645 (2.9) | … |
| Other | 6298 (6.5) | 422 (7.5) | 5876 (6.5) | … |
| Principal diagnosis, no. (%) | … | … | … | <.001§ |
| Pneumonia | 73 080 (75.7) | 4281 (76.4) | 68 799 (75.7) | … |
| Aspiration pneumonia | 3996 (4.1) | 235 (4.2) | 3761 (4.1) | … |
| Influenza pneumonia | 1883 (2.0) | 118 (2.1) | 1765 (1.9) | … |
| Viral pneumonia | 42 (0.04) | 2 (0.04) | 40 (0.04) | … |
| Sepsis | 14 216 (14.7) | 648 (11.6) | 13 568 (14.9) | … |
| Respiratory failure | 3305 (3.4) | 321 (5.7) | 2984 (3.3) | … |
| Influenza | 17 (0.02) | 1 (0.02) | 16 (0.02) | … |
| Predicted mortality on day 3, median [Q1, Q3] | 0.015 [0.007–0.028] | 0.013 [0.006–0.024] | 0.015 [0.007–0.028] | <.001† |
| Hospital characteristics | ||||
| Teaching, no. (%) | … | … | … | <.001§ |
| Yes | 31 849 (33.0) | 1994 (35.6) | 29 855 (32.8) | … |
| No | 64 690 (67.0) | 3612 (64.4) | 61 078 (67.2) | … |
| Urban/Rural, no. (%) | … | … | … | <.001§ |
| Urban | 79 804 (82.7) | 4851 (86.5) | 74 953 (82.4) | … |
| Rural | 16 735 (17.3) | 755 (13.5) | 15 980 (17.6) | … |
| Bed size, no. (%), bed category | … | … | … | <.001§ |
| ≤200 | 25 493 (26.4) | 1300 (23.2) | 24 193 (26.6) | … |
| 201–400 | 40 160 (41.6) | 2133 (38.0) | 38 027 (41.8) | … |
| ≥401 | 30 886 (32.0) | 2173 (38.8) | 28 713 (31.6) | … |
| Region, no. (%) | … | … | … | <.001§ |
| Midwest | 18 618 (19.3) | 1071 (19.1) | 17 547 (19.3) | … |
| Northeast | 17 954 (18.6) | 728 (13.0) | 17 226 (18.9) | … |
| South | 45 114 (46.7) | 2845 (50.7) | 42 269 (46.5) | … |
| West | 14 853 (15.4) | 962 (17.2) | 13 891 (15.3) | … |
| Comorbidity | ||||
| Combined comorbidity score, mean ± SD | 1.8 ± 1.8 | 1.7 ± 1.7 | 1.8 ± 1.8 | .36† |
| Anemia, no. (%) | 21 419 (22.2) | 1112 (19.8) | 20 307 (22.3) | <.001§ |
| Alcohol abuse, no. (%) | 2573 (2.7) | 183 (3.3) | 2390 (2.6) | .004§ |
| Drug abuse, no. (%) | 2426 (2.5) | 183 (3.3) | 2243 (2.5) | <.001§ |
| Chronic pulmonary disease, no. (%) | 49 032 (50.8) | 3203 (57.1) | 45 829 (50.4) | <.001§ |
| Pulmonary circulation disease, no. (%) | 6690 (6.9) | 365 (6.5) | 6325 (7.0) | .20§ |
| Valvular disease, no. (%) | 8986 (9.3) | 445 (7.9) | 8541 (9.4) | <.001§ |
| Congestive heart failure, no. (%) | 22 698 (23.5) | 1260 (22.5) | 21 438 (23.6) | .060§ |
| Diabetes, no. (%) | 10 046 (10.4) | 593 (10.6) | 9453 (10.4) | .66§ |
| Cancer, no. (%) | 2131 (2.2) | 120 (2.1) | 2011 (2.2) | .73§ |
| Paralysis, no. (%) | 1900 (2.0) | 95 (1.7) | 1805 (2.0) | .13§ |
| Obesity, no. (%) | 13 148 (13.6) | 856 (15.3) | 12 292 (13.5) | <.001§ |
| Liver disease, no. (%) | 1872 (1.9) | 123 (2.2) | 1749 (1.9) | .15§ |
| Peripheral vascular disease, no. (%) | 6721 (7.0) | 364 (6.5) | 6357 (7.0) | .16§ |
| Organ failure score, mean ± SD | 0.47 ± 0.65 | 0.44 ± 0.63 | 0.47 ± 0.66 | .001† |
| Respiratory organ failure, no. (%) | 14 224 (14.7) | 881 (15.7) | 13 343 (14.7) | .33§ |
| Cardiovascular organ failure, no. (%) | 712 (0.74) | 36 (0.64) | 676 (0.74) | .39§ |
| Renal organ failure, no. (%) | 22 449 (23.3) | 1113 (19.9) | 21 336 (23.5) | <.001§ |
| Hepatic organ failure, no. (%) | 317 (0.33) | 28 (0.50) | 289 (0.32) | .21§ |
| Metabolic organ failure, no. (%) | 2725 (2.8) | 147 (2.6) | 2578 (2.8) | .35§ |
| Neurologic organ failure, no. (%) | 4534 (4.7) | 252 (4.5) | 4282 (4.7) | .46§ |
| Multidrug resistant–community-acquired pneumonia risk factors | ||||
| Admitted from skilled nursing facility, no. (%) | 4180 (4.3) | 325 (5.8) | 3855 (4.2) | <.001§ |
| Dialysis, no. (%) | 1535 (1.6) | 72 (1.3) | 1463 (1.6) | .059§ |
| Previous admission (within 6 m), no. (%) | 4876 (5.1) | 356 (6.4) | 4520 (5.0) | <.001§ |
| Immunosuppressed, no. (%) | 9797 (10.1) | 522 (9.3) | 9275 (10.2) | .033§ |
| Initial treatments (day 1) | ||||
| Blood cultures, no. (%) | 20 662 (21.4) | 1238 (22.1) | 19 424 (21.4) | .20§ |
| Respiratory cultures, no. (%) | 3146 (3.3) | 162 (2.9) | 2984 (3.3) | .11§ |
| ICU, no. (%) | 4325 (4.5) | 317 (5.7) | 4008 (4.4) | <.001§ |
| IMV, no. (%) | 741 (0.77) | 77 (1.4) | 664 (0.73) | <.001§ |
| Non-invasive ventilation, no. (%) | 4521 (4.7) | 320 (5.7) | 4201 (4.6) | <.001§ |
| Vasopressor, no. (%) | 357 (0.37) | 17 (0.30) | 340 (0.37) | .40§ |
| Oral medication, no. (%) | 70 549 (73.1) | 4123 (73.5) | 66 426 (73.0) | .42§ |
| Initial IV antibiotics, days 0–3 | ||||
| Early third-generation cephalosporins, no. (%) | 61 482 (63.7) | 0 (0.0) | 61 482 (67.6) | <0.001§ |
| Early fluoroquinolones, no. (%) | 35 057 (36.3) | 5606 (100.0) | 29 451 (32.4) | <.001§ |
| Early macrolides, no. (%) | 61 482 (63.7) | 0 (0.0) | 61 482 (67.6) | <.001§ |
| Unadjusted outcomes | ||||
| Total duration of IV antibiotic treatment (days), median [Q1, Q3] | 4.0 [3.0–6.0] | 2.0 [1.00–2.0] | 4.0 [4.0–6.0] | <.001‡ |
| Total duration of antibiotic treatment (days), median [Q1, Q3] | 5.0 [4.0–7.0] | 4.0 [4.0–6.0] | 5.0 [4.0–,7.0] | <.001‡ |
| Late ICU (day 3+), no./total (%) | 2154/91 895 (2.3) | 91/5270 (1.7) | 2063/86 625 (2.4) | .001§ |
| Late IMV (day 3+), no./total (%) | 1295/95 585 (1.4) | 64/5524 (1.2) | 1231/90 061 (1.4) | .19§ |
| Late vasopressor (day 3+), no./total (%) | 1287/96 054 (1.3) | 68/5585 (1.2) | 1219/90 469 (1.3) | .41§ |
| Clostridium difficile infection, no./total (%) | 65/96 328 (0.07) | 2/5592 (0.04) | 63/90 736 (0.07) | .35§ |
| 14-day in-hospital mortality, no. (%) | 1199 (1.2) | 54 (0.96) | 1145 (1.3) | .052§ |
| Length of stay, median [Q1, Q3] | 4.0 [3.0–6.0] | 4.0 [3.0–5.0] | 4.0 [3.0–6.0] | <.001‡ |
| Cost,a median [Q1, Q3] | 7221.1 [5240.0–10522.5] | 6557.0 [4893.1–9462.4] | 7267.9 [5268.1–10586.3] | <.001‡ |
P values:
= Student’s t-test,
= Wilcoxon rank sum test,
= Pearson χ2 test.
Data in parenthesis are percentage of patients (%) unless otherwise indicated.
Abbreviations: ICU, intensive care unit; IMV, invasive mechanical ventilation; IV, intravenous; SD, standard deviation.
Inflation adjusted to 2015 annual costs by using the medical care component of the consumer price index.
Outcomes
After inverse propensity weighting, all variables were well balanced between early switchers and others in both the entire cohort and subgroup, with standardized differences for the propensity model variables below 10% (Supplementary Figure 1A and 1B). Table 2 summarizes the estimated effects of early switchers vs others for both the entire cohort and subgroup after propensity adjustment. For the entire cohort, early switching was associated with shorter LOS, lower hospitalization cost, fewer total days on antibiotics, less frequent late ICU admission, late IMV and late vasopressor use, and lower 14-day in-hospital case fatality. For the subgroup of patients treated with IV third-generation cephalosporins plus a macrolide or IV quinolone monotherapy, early switching was associated with shorter LOS (risk-adjusted ratio of means, 0.90; 95% confidence interval [CI], .89–.90), shorter total days on IV antibiotics (risk-adjusted ratio of means, 0.41; 95% CI, .41–.41), shorter total duration of antibiotic treatment (risk-adjusted ratio of means, 0.92; 95% CI, .92–.93), and lower hospitalization cost (risk-adjusted ratio of means, 0.89; 95% CI, .89–.90). The results retain statistical significance after Holm–Bonferroni multiple comparison adjustment. Differences in 14-day in-hospital case fatality, late ICU admission, late IMV, late vasopressor use, and incident CDI rates (Table 2) were either nonsignificant in the subgroup or did not retain significance (late ICU admissions) after multiple comparison adjustment.
Table 2.
Adjusted Outcomes Comparing Odds of Events for Patients Who Were Switched Early vs Others on Hospital Day 3; for the Entire Cohort and Among the Subgroup of Patients Initially Treated With Intravenous (IV) Ceftriaxone Plus a Macrolide or IV Quinolone Monotherapy; OR (95% Confidence Interval)
| Entire Cohort | Subgroup of Patients (Ceftriaxone + Macrolide or Quinolone Monotherapy) |
|||
|---|---|---|---|---|
| Hospitalization Outcome | Odds Ratioa | P Value | Odds Ratioa | P Value |
| 14-day in-hospital case fatality | 0.65 (.55–.77) | <.0001 | 0.77 (.54–1.11) | .16 |
| Late intensive care unit admission (hospital day 3+) | 0.66 (.58–.75) | <.0001 | 0.75 (.57–.99) | .04 |
| Late invasive mechanical ventilation initiation (hospital day 3+) | 0.67 (.57–.79) | <.0001 | 0.84 (.60–1.17) | .31 |
| Late vasopressor initiation (hospital day 3+) | 0.70 (.60–.82) | <.0001 | 0.77 (.56–1.06) | .11 |
| Clostridium difficile infection | 0.58 (.28–1.23) | 0.16 | 0.57 (.10–3.26) | .53 |
| Ratio of means | Ratio of means | |||
| Total duration of intravenous antibiotic treatment | 0.44 (.44–.44) | <.0001 | 0.41 (.41–.41) | <.0001 |
| Total duration of antibiotic treatment | 0.88 (.87–.88) | <.0001 | 0.92 (.92–.93) | <.0001 |
| Length of stay | 0.85 (.85–.86) | <.0001 | 0.90 (.89–.90) | <.0001 |
| Costb | 0.84 (.84–.84) | <.0001 | 0.89 (.89–.90) | <.0001 |
Data are odds ratios (95% confidence intervals) except for cost and length of stay (mean multipliers).
Adjusted for demographics, insurance status, comorbidities, multidrug resistant–community-acquired pneumonia risk factors, initial treatments, and disease severity (in Table 1, except for the composite organ failure score since each single organ failure was adjusted already).
Inflation adjusted to 2015 annual costs by using the medical care component of the consumer price index.
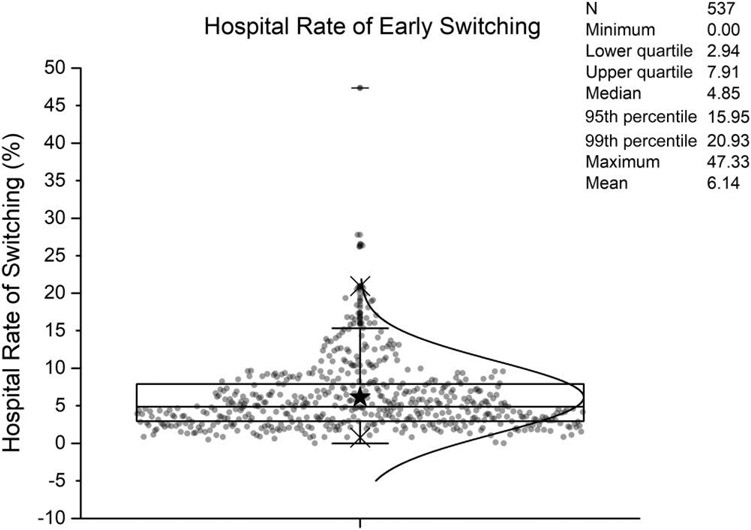
Hospital rates of early switching ranged from 0% to 47%, with a median of 4.9% (Figure 3). Table 3 shows the characteristics and outcomes of patients and hospitals across quartiles of observed switch rate. Early switching was more common in large hospitals, teaching institutions, and urban hospitals. Switching also varied across geographical regions. There were no significant differences in age (P trend = .17) and combined comorbidity scores (P trend = .66) across quartiles. Table 4 shows the adjusted outcomes of patients in hospitals across quartiles of observed switch rates. The LOS and the durations of IV and total antibiotics all declined with increasing quartile of hospital early switch rate. There were no meaningful differences in late ICU, late IMV, 14-day in-hospital case fatality, incident CDI, and hospital costs between quartiles.
Figure 3.
Superimposed dot plot and box plot of hospital fractions of community-acquired pneumonia (CAP) patients who were receiving initial intravenous (IV) antibiotics and then switched to oral antibiotics within 3 days for N = 537 hospitals with at least 100 CAP patients initially receiving IV antibiotics. Black star represents mean.
Table 3.
Characteristics and Outcomes of Patients Across Quartiles of Observed Early Switch Rate
| Hospital Rate of Early Switching | |||||
|---|---|---|---|---|---|
| Factor | <3% (N = 94 063) |
≥3% and <5% (N = 100 632) |
≥5% and <8% (N = 99 028) |
≥8% and <47% (N = 80 559) |
P Value for Trenda |
| Demographics | |||||
| Age, median [Q1, Q3], y | 74.0 [61.0–84.0] | 74.0 [61.0–84.0] | 73.0 [60.0–83.0] | 73.0 [60.0–84.0] | .14 |
| Gender, no. (%) | … | … | … | … | .01 |
| Female | 50 181 (53.3) | 52 735 (52.4) | 52 627 (53.1) | 42 243 (52.4) | … |
| Male | 43 882 (46.7) | 47 897 (47.6) | 46 401 (46.9) | 38 316 (47.6) | … |
| Race, no. (%) | … | … | … | … | <.001 |
| White | 72 262 (76.8) | 75 842 (75.4) | 74 349 (75.1) | 58 500 (72.6) | … |
| Black | 7261 (7.7) | 10 052 (10.0) | 12 804 (12.9) | 8862 (11.0) | … |
| Hispanic | 1800 (1.9) | 1038 (1.0) | 652 (0.66) | 301 (0.37) | … |
| Other | 12 714 (13.5) | 13 636 (13.6) | 11 137 (11.2) | 12 862 (16.0) | … |
| Unknown | 26 (0.03) | 64 (0.06) | 86 (0.09) | 34 (0.04) | … |
| Marital status, no. (%) | … | … | … | … | .29 |
| Married | 36 771 (39.1) | 38 153 (38.0) | 37 515 (37.9) | 29 067 (36.2) | … |
| Single | 49 986 (53.2) | 52 509 (52.3) | 49 874 (50.4) | 42 744 (53.2) | … |
| Other | 7200 (7.7) | 9824 (9.8) | 11 562 (11.7) | 8575 (10.7) | … |
| Admission source, no. (%) | … | … | … | … | .02 |
| Emergency room | 80 577 (85.7) | 90 087 (89.5) | 85 855 (86.7) | 71 684 (89.0) | … |
| SNF/ICF | 7192 (7.6) | 6588 (6.5) | 9351 (9.4) | 6292 (7.8) | … |
| Clinic | 6203 (6.6) | 3889 (3.9) | 3753 (3.8) | 2547 (3.2) | … |
| Other | 91 (0.10) | 68 (0.07) | 69 (0.07) | 36 (0.04) | … |
| Discharge disposition, no. (%) | … | … | … | … | .25 |
| Home | 45 870 (48.8) | 48 964 (48.7) | 50 132 (50.6) | 39 380 (48.9) | … |
| Home health | 15 618 (16.6) | 17 113 (17.0) | 15 772 (15.9) | 13 679 (17.0) | … |
| Hospice | 3638 (3.9) | 4138 (4.1) | 4430 (4.5) | 3569 (4.4) | … |
| Expired | 3023 (3.2) | 3023 (3.0) | 2726 (2.8) | 2430 (3.0) | … |
| Other | 25 914 (27.5) | 27 394 (27.2) | 25 968 (26.2) | 21 501 (26.7) | … |
| Insurance payer, no. (%) | … | … | … | … | .11 |
| Medicare | 68 875 (73.2) | 73 593 (73.1) | 72 375 (73.1) | 59 132 (73.4) | … |
| Medicaid | 6971 (7.4) | 8201 (8.1) | 7461 (7.5) | 6497 (8.1) | … |
| Managed care | 10 534 (11.2) | 11 240 (1 1.2) | 10 480 (10.6) | 7791 (9.7) | … |
| Commercial indemnity | 2699 (2.9) | 1989 (2.0) | 3013 (3.0) | 2306 (2.9) | … |
| Other | 4984 (5.3) | 5609 (5.6) | 5699 (5.8) | 4833 (6.0) | … |
| Principal diagnosis, no. (%) | … | … | … | … | .017 |
| Pneumonia | 60 006 (63.8) | 59 531 (59.2) | 60 575 (61.2) | 47 144 (58.5) | … |
| Aspiration pneumonia | 9182 (9.8) | 10 257 (10.2) | 9694 (9.8) | 8221 (10.2) | … |
| Influenza pneumonia | 1389 (1.5) | 1573 (1.6) | 1456 (1.5) | 1212 (1.5) | … |
| Viral pneumonia | 28 (0.03) | 39 (0.04) | 29 (0.03) | 63 (0.08) | … |
| Sepsis | 20 292 (21.6) | 25 956 (25.8) | 23 298 (23.5) | 21 122 (26.2) | … |
| Respiratory failure | 3152 (3.4) | 3264 (3.2) | 3965 (4.0) | 2783 (3.5) | … |
| Influenza | 14 (0.01) | 12 (0.01) | 11 (0.01) | 14 (0.02) | … |
| Predicted mortality on day 3 | 0.03 ± 0.04 | 0.03 ± 0.04 | 0.03 ± 0.04 | 0.03 ± 0.04 | .27 |
| Hospital characteristics | |||||
| Teaching, no. (%) | 22 545 (24.0) | 38 039 (37.8) | 34 055 (34.4) | 35 884 (44.5) | <.001 |
| Yes | 71 518 (76.0) | 62 593 (62.2) | 64 973 (65.6) | 44 675 (55.5) | … |
| No | … | … | … | … | … |
| Urban/Rural, no. (%) | 76 400 (81.2) | 79 548 (79.0) | 86 603 (87.5) | 72 758 (90.3) | <.001 |
| Urban | 17 663 (18.8) | 21 084 (21.0) | 12 425 (12.5) | 7801 (9.7) | … |
| Rural | … | … | … | … | … |
| Bed size, no. (%), bed category | 27 527 (29.3) | 26 477 (26.3) | 19 677 (19.9) | 15 778 (19.6) | <.001 |
| ≤200 | 44 591 (47.4) | 32 633 (32.4) | 43 455 (43.9) | 29 210 (36.3) | … |
| 201–400 | 21 945 (23.3) | 41 522 (41.3) | 35 896 (36.2) | 35 571 (44.2) | … |
| ≥401 | |||||
| Region, no. (%) | 19 685 (20.9) | 14 782 (14.7) | 21 186 (21.4) | 15 011 (18.6) | <.001 |
| Midwest | 17 785 (18.9) | 20 266 (20.1) | 11 258 (11.4) | 12 027 (14.9) | … |
| Northeast | 49 691 (52.8) | 46 675 (46.4) | 55 837 (56.4) | 32 052 (39.8) | … |
| South | 6902 (7.3) | 18 909 (18.8) | 10 747 (10.9) | 21 469 (26.7) | … |
| West | 22 545 (24.0) | 38 039 (37.8) | 34 055 (34.4) | 35 884 (44.5) | … |
| Multidrug resistant-community-acquired pneumonia risk factors | |||||
| Admission within last 6 mo, no. (%) | 10 973 (11.7) | 10 967 (10.9) | 10 757 (10.9) | 8476 (10.5) | <.001 |
| Dialysis, no. (%) | 3450 (3.7) | 4043 (4.0) | 3783 (3.8) | 2604 (3.2) | <.001 |
| Admitted from SNF/ICF, no. (%) | 7192 (7.6) | 6588 (6.5) | 9351 (9.4) | 6292 (7.8) | <.001 |
| Comorbidity | |||||
| Combined comorbidity scores, median [Q1, Q3] | 2.1 ± 2.0 | 2.1 ± 2.0 | 2.1 ± 2.0 | 2.1 ± 2.0 | .99 |
| Hypertension, no. (%) | 63 859 (67.9) | 67 290 (66.9) | 67 301 (68.0) | 52 918 (65.7) | <.001 |
| Chronic pulmonary disease, no. (%) | 47 244 (50.2) | 47 781 (47.5) | 48 504 (49.0) | 39 304 (48.8) | .026 |
| Anemia, no. (%) | 27 251 (29.0) | 28 272 (28.1) | 27 870 (28.1) | 22 346 (27.7) | <.001 |
| Diabetes, no. (%) | 12 502 (13.3) | 12 966 (12.9) | 12 994 (13.1) | 10 428 (12.9) | .17 |
| Congestive heart failure, no. (%) | 25 982 (27.6) | 27 328 (27.2) | 26 463 (26.7) | 21 270 (26.4) | <.001 |
| Treatment/tests on day 1 | |||||
| Blood cultures, no. (%) | 19 141 (20.3) | 16 933 (16.8) | 27 948 (28.2) | 14 346 (17.8) | <.0001 |
| Respiratory cultures, no. (%) | 3520 (3.7) | 2733 (2.7) | 3886 (3.9) | 2175 (2.7) | <.001 |
| ICU, no. (%) | 7885 (8.4) | 9899 (9.8) | 9521 (9.6) | 8657 (10.7) | <.001 |
| IMV, no. (%) | 1325 (1.4) | 1785 (1.8) | 1502 (1.5) | 1560 (1.9) | <.001 |
| NIV, no. (%) | 6637 (7.1) | 6390 (6.3) | 6271 (6.3) | 5373 (6.7) | .10 |
| Vasopressor, no. (%) | 1425 (1.5) | 1567 (1.6) | 1565 (1.6) | 1459 (1.8) | <.001 |
| Oral medication, no. (%) | 64 980 (69.1) | 70 015 (69.6) | 71 127 (71.8) | 57 010 (70.8) | <.001 |
| Unadjusted outcomes | |||||
| Total duration of intravenous antibiotic treatment (days), median [Q1, Q3] | 5.0 [4.0–8.0] | 5.0 [4.0–7.0] | 5.0 [4.0–7.0] | 4.0 [3.0–6.0] | <.001 |
| Total duration of antibiotic treatment (days), median [Q1, Q3] | 6.0 [4.0–8.0] | 6.0 [4.0–8.0] | 6.0 [4.0–7.5] | 5.0 [4.0–7.0] | <.001 |
| Late ICU (day 3+), no./total (%) | 2819/85 685 (3.3) | 3110/90 117 (3.5) | 2969/88 886 (3.3) | 2374/71 307 (3.3) | .25 |
| Late IMV (day 3+), no./total (%) | 1865/92 382 (2.0) | 2054/98 514 (2.1) | 1998/97 230 (2.1) | 1474/78 768 (1.9) | .04 |
| Late vasopressor (day 3+), no./total (%) | 1954/92 289 (2.1) | 1993/98 677 (2.0) | 2087/97 052 (2.2) | 1364/78 771 (1.7) | <.001 |
| Clostridium difficile infection, no./total (%) | 107/90 974 (0.12) | 100/95 997 (0.10) | 92/95 430 (0.10) | 76/77 614 (0.10) | .19 |
| 14-day in hospital mortality, no. (%) | 2382 (2.5) | 2448 (2.4) | 2184 (2.2) | 2019 (2.5) | .78 |
| Length of stay, median [Q1, Q3] | 5.0 [4.0–7.0] | 5.0 [4.0–7.0] | 5.0 [4.0–7.0] | 5.0 [3.0–7.0] | <.001 |
| Cost,b median [Q1, Q3] | 8825.9 [6096.4–13439.9] | 8505.2 [5862.9–12922.7] | 8208.4 [5824.6–12297.9] | 8568.1 [6033.5–12752.4] | <.25 |
P for trend for continuous and binary variables only.
Inflation adjusted to 2015 annual costs by using the medical care component of the consumer price index.
Abbreviations: ICF, intermediate care facility; ICU, intensive care unit; IMV; invasive mechanical ventilation; SNF, skilled nursing facility.
Table 4.
Adjusted Outcomes of Patients Across Quartiles of Observed Early Switch Rate; OR 95% Confidence Interval
| Hospital Rate of Early Switching | ||||
|---|---|---|---|---|
| <3% (N = 94 063) |
≥3% and <5% (N = 100 632) |
≥5% and <8% (N = 99 028) |
≥8% and <47% (N = 80 559) |
|
| Hospitalization Outcome | Base Category | Odds Ratio | ||
| 14-day in-hospital case fatality | N/A | 1.01 (.89–1.13) | 0.96 (.85–1.08) | 1.08 (.96–1.23) |
| Late intensive care unit admission (hospital day 3+) | N/A | 1.08 (.96–1.22) | 0.99 (.88–1.12) | 1.04 (.91–1.18) |
| Late invasive mechanical ventilation initiation (hospital day 3+) | N/A | 1.06 (.90–1.24) | 1.03 (.88–1.20) | 0.99 (.84–1.17) |
| Late vasopressor initiation (hospital day 3+) | N/A | 0.97 (.83–1.14) | 0.98 (.84–1.15) | 0.80 (.68–.95) |
| Clostridium difficile infection | N/A | 0.96 (.69–1.35) | 0.84 (.60–1.18) | 0.92 (.64–1.31) |
| Ratio of means | ||||
| Total duration of intravenous antibiotic treatment | N/A | 0.94 (.92–.96) P < .0001 |
0.90 (.88–.92) P < .0001 |
0.80 (.78–.82) P < .0001 |
| Total duration of antibiotic treatment, Median | N/A | 0.97 (.95–.99) P = .0052 |
0.95 (.93–.97) P < .0001 |
0.91 (.89–0.93) P < .0001 |
| Length of stay | N/A | 0.98 (.95–1.003 P = .0955 |
0.96 (.94–.99) P = .0031 |
0.93 (.90–.95) P < .0001 |
| Costa | N/A | 0.95 (.83–1.08) P = .4348 |
0.93 (.81–1.06) P = .2676 |
0.93 (.81–1.07) P = .3051 |
Abbreviation: N/A, Not Applicable.
Inflation adjusted to 2015 annual costs by using the medical care component of the consumer price index
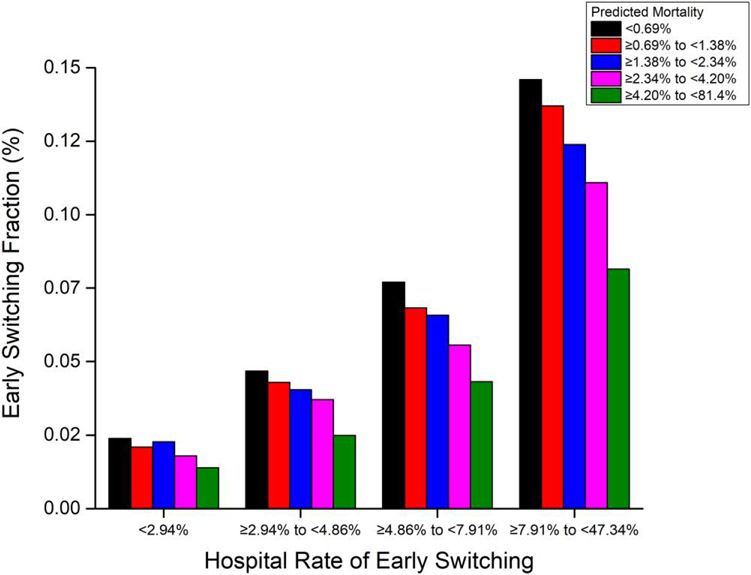
Figure 4 shows switch rates for patients within each hospital quartile, stratified by quintile of predicted mortality. Within each switch rate quartile, patients at higher predicted mortality risk were less likely to be switched. However, high-risk patients at hospitals with high switch rates were more than 3 times as likely to be switched as low-risk patients at hospitals with low switch rates. Even in hospitals in the upper switching quartile, the switch rate among patients at the lowest mortality risk was <15%.
Figure 4.
Fractions of community-acquired pneumonia (CAP) patients who were receiving initial intravenous antibiotics (at N = 537 hospitals with at least 100 patients) and then switched to oral antibiotics within 3 days, stratified by patient predicted mortality (colored bars), grouped by hospital early switching rate quartile (horizontal axis). Early switching appears to be more strongly guided by hospital practices than by patient prognosis.
DISCUSSION
In a large nationally representative cohort of patients with CAP treated with IV antibiotics, <30% of patients were switched to oral antibiotics before discharge and <6% were switched early. Patients who were switched early were less sick (more likely to have a diagnosis of pneumonia and less likely to have a diagnosis of sepsis) and had fewer comorbidities than those who were switched later. Early switching was associated with fewer days on IV antibiotics, shorter total duration of antibiotics, and shorter LOS but not with worse outcomes. Hospital practices varied; large urban hospitals, which often have the sickest patients, had the highest rates of early switching. However, even in hospitals in the upper quartile of early switch rates, clinical outcomes were similar while duration of antibiotics and LOS were shorter.
Multiple previous studies have demonstrated the safety of early switching in patients with CAP. In a multicenter randomized trial of 265 patients in non-ICUs with severe CAP published in 2006, Oosterheert et al reported that early switching was safe (no differences in 28-day mortality and clinical cure rates) and decreased the hospital LOS by 2 days [9]. A 2008 meta-analysis (6 randomized, controlled trials, n = 1219) found that early switching was safe and decreased hospital LOS without adversely affecting patient outcomes [15]. In another randomized, controlled trial (n = 401), Carratala et al reported that use of a 3-step pathway (early mobilization, using objective criteria for switching therapy, and determining hospital discharge) was safe and helped reduce the duration of IV antibiotic therapy and LOS in patients with CAP [20]. However, together, these studies enrolled fewer than 2000 patients and were underpowered to detect important increases in mortality or late deterioration. Our study confirms these findings in a much larger, more recent, multihospital cohort.
Despite the evidence for the safety of early switching, relatively few patients in our study were switched by hospital day 3, and most received IV therapy throughout their stays. Physicians were some-what sensitive to risk, with patients at lower predicted mortality more often switched early. However, the choice of initial antibiotic was crucial. Only patients who were started on quinolones were switched early, likely because oral quinolones have excellent bioavailability. The overwhelming majority of low-risk patients were not switched, highlighting an opportunity to encourage clinicians to follow evidence-based guidelines to reduce both IV and total antibiotics administered to clinically stable patients.
Our study has limitations. First, by relying on ICD-9 coding, we may have misclassified some patients with respect to CAP and some potential confounders. Second, although we adjusted for potential baseline confounders and mortality risk on hospital day 3, it is likely that there were unmeasured or other confounders at the time of switching for which we could not adequately account. Specifically, we did not have access to clinical data such as vital signs or progress notes and therefore could not directly assess clinical stability or severity. However, we used indirect markers of clinical severity on hospital day 3 that were previously shown to have good prognostic ability. Third, in our study, only patients started on fluoroquinolones were switched early; therefore, we cannot draw any conclusions about the outcomes of nonfluoroquinolone antibiotics. Last, our study findings are in agreement with previous studies; we used data from 2010–2015, and some of our results may not reflect current antimicrobial practices.
Early switching appears safe but is underused in patients with CAP. Only a small fraction of eligible patients were switched by hospital day 3. Hospital switch rates varied widely and were low even among patients at low mortality risk. Our data suggest hospitals can reduce the burden of antibiotics delivered for CAP by encouraging clinicians to follow evidence-based recommendations to switch therapy in clinically stable patients.
Supplementary Material
Financial support.
This work was supported by the Agency for Healthcare Research and Quality (grant R01 HS024277-01A1; M. B. R., A.D., S. H., P. K. L., N.G., M. D. Z., T.H., and P. B. I.). A. D. was supported by the Agency for Healthcare Research and Quality under award K08 HS025026. M. D. Z. reports consulting fees in support of this work from the Cleveland Clinic.
Footnotes
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Disclaimer. The funding agency had no role in the design or conduct of the study or in the drafting of the manuscript.
Conflict of interest. A. D. reports research funding (to institution) from the Clorox Company, consultant fees from Merck, and grants or contracts to institution, unrelated to the current study, from Seres Therapeutics. M. K. reports royalties from UpToDate and grants or contracts to institution from the Centers for Disease Control and Prevention and the Agency for Healthcare Research and Quality. M. D. Z. reports research support from Tetraphase Pharmaceuticals, Astellas, Lungpacer, Merck, Spero, Medicines Co, Melinta, scPharma, Shionogi, and Pfizer; consulting fees from Paratek, Arasanis, Shionogi, Pfizer, Nabriva, scPharma, and Melinta; and stock or stock options from Johnson & Johnson. T. H. reports consulting fees from the Cerner Corporation. P. B. I. reports consulting fees from Colgate-Palmolive. S. H. reports a role with the Society for Health Epidemiology of America (councilor, board of trustees, volunteer; no compensation). All remaining authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Najafi S, Sandrock C. Hospitalized patients with acute pneumonia. Hosp Med Clin 2017; 6:456–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams S, Gousen S, DeFrances C. National Hospital Care Survey demonstration projects: pneumonia inpatient hospitalizations and emergency department visits. Natl Health Stat Report 2018; 116:1–11. [PubMed] [Google Scholar]
- 3.Brown K, Valenta K, Fisman D, Simor A, Daneman N. Hospital ward antibiotic prescribing and the risks of Clostridium difficile infection. JAMA Intern Med 2015; 175:626–33. [DOI] [PubMed] [Google Scholar]
- 4.Tacconelli E, De Angelis G, Cataldo MA, et al. Antibiotic usage and risk of colonization and infection with antibiotic-resistant bacteria: a hospital population-based study. Antimicrob Agents Chemother 2009; 53:4264–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yi SH, Hatfield KM, Baggs J, et al. Duration of antibiotic use among adults with uncomplicated community-acquired pneumonia requiring hospitalization in the United States. Clin Infect Dis 2018; 66:1333–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laing RB, Mackenzie AR, Shaw H, Gould IM, Douglas JG. The effect of intravenous-to-oral switch guidelines on the use of parenteral antimicrobials in medical wards. J Antimicrob Chemother 1998; 42:107–11. [DOI] [PubMed] [Google Scholar]
- 7.Omidvari K, de Boisblanc BP, Karam G, Nelson S, Haponik E, Summer W. Early transition to oral antibiotic therapy for community-acquired pneumonia: duration of therapy, clinical outcomes, and cost analysis. Respir Med 1998; 92:1032–9. [DOI] [PubMed] [Google Scholar]
- 8.Marrie TJ, Lau CY, Wheeler SL, Wong CJ, Vandervoort MK, Feagan BG. A controlled trial of a critical pathway for treatment of community-acquired pneumonia. CAPITAL Study Investigators. Community-acquired pneumonia intervention trial assessing levofloxacin. JAMA 2000; 283:749–55. [DOI] [PubMed] [Google Scholar]
- 9.Oosterheert JJ, Bonten MJ, Schneider MM, et al. Effectiveness of early switch from intravenous to oral antibiotics in severe community acquired pneumonia: multicentre randomised trial. BMJ 2006; 333:1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramirez JA, Vargas S, Ritter GW, et al. Early switch from intravenous to oral antibiotics and early hospital discharge: a prospective observational study of 200 consecutive patients with community-acquired pneumonia. Arch Intern Med 1999; 159:2449–54. [DOI] [PubMed] [Google Scholar]
- 11.Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis 2016; 62:e51–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007; 44(Suppl 2):S27–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engel MF, Postma DF, Hulscher ME, et al. Barriers to an early switch from intravenous to oral antibiotic therapy in hospitalised patients with CAP. Eur Respir J 2013; 41:123–30. [DOI] [PubMed] [Google Scholar]
- 14.Broom J, Broom A, Adams K, Plage S. What prevents the intravenous to oral antibiotic switch? A qualitative study of hospital doctors’ accounts of what influences their clinical practice. J Antimicrob Chemother 2016; 71:2295–9. [DOI] [PubMed] [Google Scholar]
- 15.Athanassa Z, Makris G, Dimopoulos G, Falagas ME. Early switch to oral treatment in patients with moderate to severe community-acquired pneumonia: a meta-analysis. Drugs 2008; 68:2469–81. [DOI] [PubMed] [Google Scholar]
- 16.Moore BJ, White S, Washington R, Coenen N, Elixhauser A. Identifying increased risk of readmission and in-hospital mortality using hospital administrative data: the AHRQ Elixhauser comorbidity Index. Med Care 2017; 55:698–705. [DOI] [PubMed] [Google Scholar]
- 17.Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol 2011; 64:749–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walkey AJ, Shieh MS, Liu VX, Lindenauer PK. Mortality measures to profile hospital performance for patients with septic shock. Crit Care Med 2018; 46:1247–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rothberg MB, Pekow PS, Priya A, et al. Using highly detailed administrative data to predict pneumonia mortality. PLoS One 2014; 9:e87382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carratala J, Garcia-Vidal C, Ortega L, et al. Effect of a 3-step critical pathway to reduce duration of intravenous antibiotic therapy and length of stay in community-acquired pneumonia: a randomized controlled trial. Arch Intern Med 2012; 172:922–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.