Figure 3.
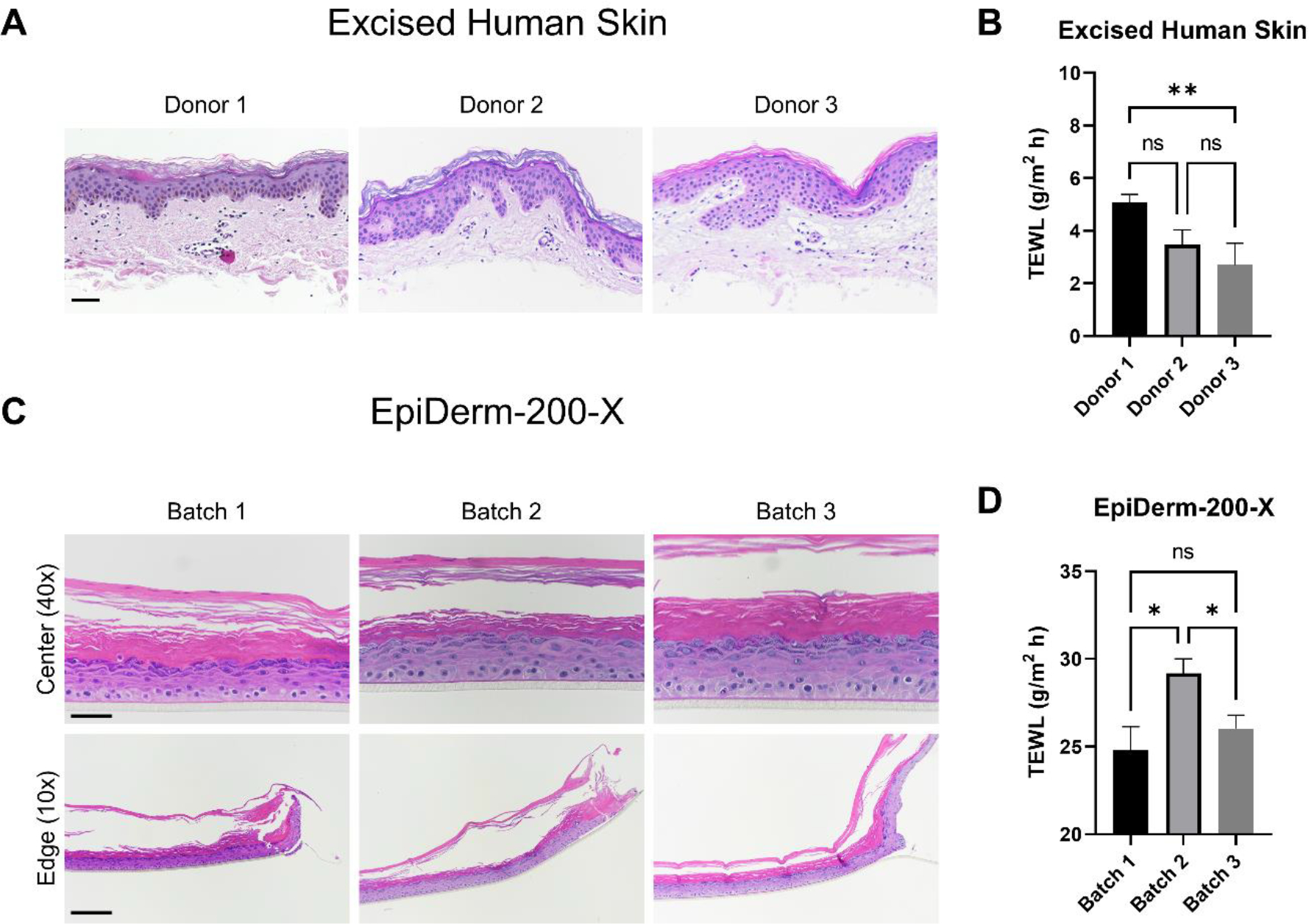
Assessment of excised human skin (EHS) and EpiDerm-200-X reconstructed human epidermis morphological and barrier variability amongst donors/batches. EHS samples from three donors were fixed and processed for histological examination (A). Samples were stained with hematoxylin and eosin (H&E). Representative images are presented. Scale bar = 50 μm. Transepidermal water loss (TEWL) was measured on EHS samples mounted on 5 mm FCs (B). N = 6 – 20 samples per donor. The difference in mean TEWL was statistically compared by ANOVA with Tukey’s test; ns = no significance, ** = p < 0.01. Three independently received EpiDerm-200-X batches were examined by histology (H&E; C) and TEWL (D) in an identical manner as EHS. Representative H&E images are presented. 40X scale bar = 50 μm; 10X scale bar = 200 μm. ‘Center’ and ‘Edge’ denote the portion of the tissue closest to the center or the edge of the transwell support upon which it was manufactured, respectively. N = 8 – 22 samples per batch. Data shown as mean ± standard error of mean. The difference in mean TEWL was statistically compared by ANOVA with Tukey’s test; ns = no significance, * = p < 0.05.
