Abstract
Since the publication of the last Cellular Therapy and Stem Cell Transplant blueprint in 2013, Children’s Oncology Group cellular therapy-based trials for advanced the field and created new standards of care across a wide spectrum of pediatric cancer diagnoses. Key findings include that tandem autologous transplant improved survival for patients with neuroblastoma and atypical teratoid/rhabdoid brain tumors, one umbilical cord blood (UCB) donor was safer than two UCB donors, killer immunoglobulin receptor (KIR) mismatched donors did not improve survival for pediatric acute myeloid leukemia when in vivo T cell depletion is used and the depth of remission as measured by next-generation sequencing based minimal residual disease assessment pre-transplant was the best predictor of relapse for acute lymphoblastic leukemia. Plans for the next decade include optimizing donor selection for transplants for acute leukemia/myelodysplastic syndrome, using novel engineered cellular therapies to target a wide array of malignancies, and developing better treatments for cellular therapy toxicities such as viral infections and graft-vs-host disease.
Introduction:
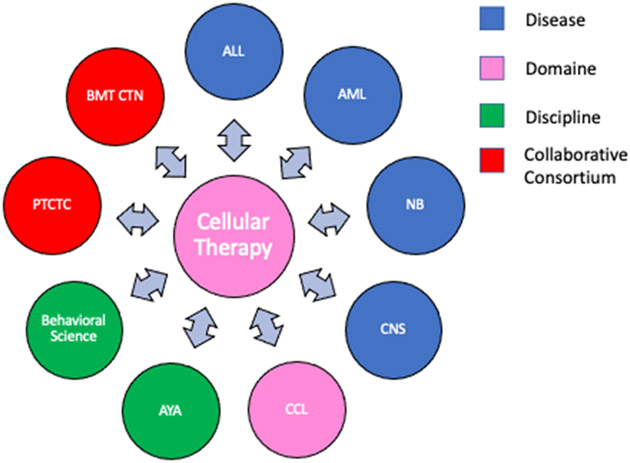
The Cellular Therapy and Stem Cell Transplantation (CT) committee is a domain within the Children’s Oncology Group (COG) and as such develops its own trials in addition to collaborating with other diseases committees, domains, and disciplines as well as external groups such as the Pediatric Transplant and Cellular Therapy Consortium (PTCTC) and the Blood and Marrow Transplant Clinical Trials Network (BMT CTN; Figure 1) in areas related to transplant and cellular therapy for pediatric cancer. For some COG studies, the primary research question concerns cellular therapy practice such as the best donor source, e.g., ASCT2031, haploidentical vs unrelated donor), preparative regimen intensity for juvenile myelomonocytic leukemia (JMML) (ASCT1221), or whether the inclusion of sirolimus in graft-vs-host disease (GVHD) prophylaxis can improve outcomes for patients with minimal residual disease (MRD) prior to transplant (ASCT0431). Such studies are best run by the CT committee directly; in other cases, the cellular therapy component may be one block of a larger treatment plan for specific diseases, such as ACNS0333 and ANBL0532 (tandem autologous transplant as consolidation following induction chemotherapy for brain tumors and neuroblastoma respectively). Studies focused on supportive care may be more appropriate for the Cancer Control and Supportive Care domain portfolio, such as ACCL0934 or ACCL1131, testing different infection prophylaxis strategies early post-HCT.
Figure 1:
The collaborative network of the Cellular Therapy Committee of the Children’s Oncology Group. Abbreviations: ALL – Acute lymphoblastic leukemia, AML – Acute myelogenous leukemia, NB – neuroblastoma, CNS – Central nervous system, CCL – Cancer Control, AYA – Adolescent and young adult, PTCTC – Pediatric Transplant and Cellular Therapy Consortium, BMT CTN – Bone marrow transplant clinical trials network.
The hematopoietic cell transplantation (HCT) field has seen major advancements over the past decade such as immune effector cell therapy (e.g., CD19-directed chimeric antigen receptor T cells [CAR T-cells] for B-cell malignancies) and hematopoietic stem cell gene therapy for hemoglobinopathies.1-3 As a result, many transplant programs, national organizations, and journals changed their names to be more inclusive of these emerging treatments. The former COG Stem Cell Transplant Committee was renamed the Cellular Therapy (CT) Committee to reflect this evolution in the field. Looking to the future, COG’s strong record of accomplishment in studying rare diseases is ideally suited to test emerging immune based cellular therapies for multiple hematologic and solid cancers in children and to treat post-HCT complications such as GVHD and refractory viral infections among others.
Most pediatric transplant and cellular therapy programs and smaller consortia treat small numbers of patients. International cooperative groups such as COG are necessary to conduct pivotal trials that require larger numbers of patients to move the field forward. The CT Committee’s three overarching goals are: 1) to improve the efficacy of CT (allogeneic, autologous and immune effector cells) in childhood cancer; 2) to improve the safety of CT; and 3) to optimize design, collaborate in the development, and assure timely completion of CT-related treatment and research protocols developed through COG disease and domain committees.
Key Achievements Since 2013 Blueprint:
Transplant Specific Trials (Table 1):
Table 1:
Cellular Therapy Specific Trials
| First Author |
COG Study/Collaboration |
Reference | Findings | Outcome/Follow-up Study |
|---|---|---|---|---|
| Yanik | ASCT0521 | 57 | Phase 2 study of TNF blockade with etanercept for the treatment of idiopathic pneumonia syndrome (IPS). 28 IPS patients were treated and a high rate of complete responses, 71%. | Etanercept is standard of care for pediatric IPS |
| Pulsipher | ASCT 0431 – Ancillary analysis | 7 | IgH-V(D)J NGS-MRD predicted relapse and survival post-HCT better than flow MRD. Relapse 0 vs 16%, 2 yr OS 96% vs 77% | PTCTC ONC1701 (EndRAD Trial) to test radiation free conditioning in NGS MRD- patients |
| Wagner | BMT CTN 0501 | 17 | Survival similar for both single vs double cord blood transplants (73% vs 65%) but better platelet recovery and less grade 3-4 GVHD with single cord | Single cord blood unit HCT preferred for children |
| Dvorak | ASCT1221 | 13 | Tested BuCyMel vs BuFlu regimens in JMML patients. More complications with BuCyMel but more relapses with BuFlu. | BuCyMel remains standard of care despite more complications |
ASCT0431:
This randomized trial of sirolimus-based GVHD prophylaxis after HCT for acute lymphoblastic leukemia (ALL) closed after an interim analysis of the first 145 patients determined the primary endpoint, improved survival, would not be met.4 However, correlative studies have proved a rich resource that informed subsequent studies.5, 6 First, GVHD was protective against relapse (hazard ratio (HR), 0.4; p=0.04), demonstrating the correlation of GVHD with the graft-vs-leukemia (GVL) effect in pediatric ALL. Second, the threshold for MRD by multichannel flow cytometry-based MRD (MFC-MRD) pre-HCT for increased risk of relapse was established at ≥0.1% (HR, 3.3; p=0.01). Third, precise MRD testing using next-generation sequencing (NGS) pre-HCT based on immunoglobulin heavy chain (IgH)-variable, diversity, and joining (V[D]J) or T-cell receptor clonal rearrangements (NGS-MRD) was the best predictor for relapse. Patients who were NGS-MRD(−) pre-HCT had significantly lower 2-year relapse rates compared to patients who were NGS-MRD(+) (0% vs 53%, p<0.001). Furthermore, patients who were NGS-MRD(−) pre-HCT were less likely to relapse than patients who were MFC-MRD(−); (0% vs 16%, p=0.02) and more likely to survive (96% vs 77%, p=0.004).4, 7 The exceptionally low rate of relapse in patients NGS-MRD(−) pre-HCT led to a trial testing a novel, potentially less toxic preparative regimen for patients with pediatric ALL in deep remission. Total body irradiation (TBI)-based preparative regimens for pediatric ALL reduce relapse compared to chemotherapy-based preparative regimens but at the cost of more short- and long-term toxicity.8, 9 PTCTC trial NCT03509961 is testing whether a novel radiation-free regimen is as effective as TBI-based regimens for NGS-MRD(−) ALL patients.
ASCT1221:
Allogeneic HCT is the only current curative option for patients with JMML but needs improvement given high rates of both relapse and treatment complications. Challenges with HCT for JMML include young age at diagnosis (<2 years), high disease burden that does not respond well to chemotherapy, and morbidities such as massive hepatosplenomegaly and pulmonary infiltrates.10 A busulfan-cyclophosphamide-melphalan (Bu-Cy-Mel) preparative regimen is the standard of care but transplant related mortality (TRM) rates approach 20%.11, 12 ASCT1221 tested if a less toxic preparative regimen, busulfan-fludarabine (Bu-Flu), would provide disease control with less mortality. Fifteen patients were randomized to either Bu-Cy-Mel (n=6) or Bu-Flu (n=9). There were fewer cases of veno-occlusive disease/sinusoidal obstructive syndrome (VOD/SOS) on the Bu-Flu arm (2/9, 22%) compared to the Bu-Cy-Mel arm (3/6, 50%), but the study was closed early based on large differences in 18 month event-free survival (EFS) favoring Bu-Cy-Mel (83% vs 22%), although this did not reach statistical significance.13
BMT CTN 0501:
This joint collaboration between COG and the BMT CTN tested the hypothesis that 1-year survival would be better for pediatric patients transplanted with two partially human leukocyte antigen (HLA)-matched umbilical cord blood units UCB units compared to one UCB unit based on studies showing better survival in adults transplanted two UCB units14-16. Eligible patients (≤21 years, had a high-risk hematologic malignancy, and two adequately matched (≥4/6 HLA-match for A, B, and DR) UCB units, where at least one unit had a cell dose of ≥2.5 X 107 nucleated cells/kg recipient body weight) were randomly assigned to transplant with either two UCB (n=111) or one UCB (n=113) unit. All patients received the same TBI-based preparative regimen and GVHD prophylaxis strategy. One year overall survival (OS) from the time of randomization was not different between the two vs one UCB unit arms (65% vs 73%, p=0.17). There were also no statistically different rates of disease-free survival (DFS), relapse, TRM, neutrophil recovery and rates of grade II-IV acute GVHD. Patients transplanted with one UCB recovered platelet counts earlier (58 vs 84 days, p=0.04) and experienced less grade III-IV acute GVHD (13% vs 23%, p=0.02).17 Single UCB unit is the standard of care for cord blood HCT in children given the shorter hematologic recovery time, lower risk of severe acute GVHD, and cost-savings.
Collaborations with Solid Tumor Committees (Table 2):
Table 2:
Collaborations with other COG committees
| First Author |
COG Study | Reference | Findings | Outcome/Follow-up Study |
|---|---|---|---|---|
| Pollard | AAML1031 | 31 | Sorafenib for HAR FLT3/ITD+ patients during chemo and 1 yr of maintenance (post-HCT if donor available). Sorafenib exposed patients had improved EFS, DFS and relapse rates. | AAML1831 – Test gilteritinib, a second generation TKI, that may be less toxic than sorafenib. |
| Davies | AAML05P1 | 28 | Prospective study of KIR typing of unrelated donors prior to donor selection for patients with AML. No difference in OS, DFS or RR between KIR matched and mismatched. Acute GVHD rates were lower in the mismatched group. | PTCTC ONC1401 trial – testing whether KIR mismatches are beneficial with haploidentical donors |
| Reddy | ACNS0333 | 24 | Intensified treatment for CNS AT/RT patients improved 4 yr EFS from 6% to 35%. | Planned ACNS2232 – add tazemetostat to ACNS0333 schema to decrease relapse. |
| Park | ANBL0532 | 21 | Randomized 355 HR NB to 1 (CEM) vs 2 (Thio/Cy then dose reduced CEM) HCT. Tandem recipients had improved 3 yr EFS 62% vs 48%. | ANBL1531 – testing whether 131I-MIBG treatment during induction improves EFS. |
| Andolina | AYA | 36 | Retrospective analysis of GVHD, relapse, and survival in 188 pts transplanted on 3 COG protocols for acute leukemia (ASCT0431, AAML0531, AAML1031). Compared rates of acute and chronic GVHD, relapse and survival based on younger child (ages 2-12) vs AYA (13-21). AYA were more likely to experience grade II-IV acute GVHD compared to younger children (56% vs 32%, p=0.006) and less likely to relapse (20% vs 35%, p=0.032). No difference by age for chronic GVHD (26% vs 20%, p=0.72), EFS (43% vs 52%, p=0.18) or OS (49% vs 52%, p=0.56). | These results formed the basis of the age stratification in the current ASCT2031 study. |
| Schofield | 58 | Joint Cellular Therapy and Behavioral Sciences task force developed evidence based guidelines for monitoring neurocognitive outcomes following cellular therapy treatments. | Guidelines inform COG CT trial design. |
ANBL0532:
High-risk neuroblastoma outcomes have improved from <10% to 40-50% as intensity of treatment increased.18 Current multi-modal treatment includes a complex sequence of multi-agent chemotherapy, surgery, high dose chemotherapy followed by autologous HCT consolidation, post-HCT radiation, and finally several cycles of biologic and immune therapies. Intensification of the treatment with tandem autologous HCT further improved outcomes in pilot studies.19, 20 ANBL0532 tested this approach in a randomized trial of single (n=179) vs tandem (n=176) HCT. Patients randomized to single HCT received conditioning with standard dose carboplatin-etoposide-melphalan (CEM). Patients in the tandem HCT group received cyclophosphamide and thiotepa for the first HCT followed by dose reduced CEM for the second HCT after hematopoietic and organ recovery. Three year EFS from the time of randomization was superior for tandem over single HCT (62% vs 48%, p=0.006) without more toxicity and thus established a new standard of care.21 The current COG protocol, ANBL1531, uses tandem HCT consolidation and tests the hypothesis that therapeutic 131I-meta-iodobenzylguanidine (131I-MIBG) during induction will improve outcomes. ANBL1531 also tested whether a single HCT using dose-intensive Busulfan-Melphalan (Bu-Mel) would be as safe and effective as the tandem HCT approach based on encouraging results.22, 23 However, the Bu-Mel arm was inferior to tandem HCT on interim analysis and closed in December 2020. ANLB1531 should complete enrollment in 2023.
ACNS0333:
Atypical teratoid/rhabdoid tumor (AT/RT) is a rare, highly aggressive brain cancer typically diagnosed in infants and toddlers with exceptionally poor survival rates <10%. ACNS0333, the first cooperative group trial for AT/RT, utilized dose intensification including two induction cycles of vincristine, high dose methotrexate, etoposide, cyclophosphamide and cisplatin followed by three sequential cycles of high dose, sub-ablative carboplatin and thiotepa supported by autologous HCT (to hasten marrow recovery and shorten the interval between courses) and then consolidation with involved field radiation. Four-year EFS was higher for study patients (n=68) than historic controls (35% vs 6%, p<0.0005) without any treatment-related deaths or secondary malignancies despite high rates of hematologic and infectious toxicities. Relapse was the major cause for treatment failure.24 Patients with AT/RT have biallelic alterations of the SMARCB1 gene that decreases SMARCB1 protein expression and results in EZH2 overexpression. A phase 1b trial showed that tazemetostat, an oral EZH2 inhibitor, induced responses in relapsed AT/RT.25 ACNS2232 is study in development to test whether the addition of tazemetostat to the ACNS0333 schema followed by tazemetostat maintenance therapy decreases relapse.
Collaborations with Hematologic Malignancy Committees:
AAML05P1:
Relapse and GVHD rates are lower following ex vivo T-cell depleted HCT from KIR mismatched donors.26 The impact of KIR mismatch without T-cell depletion has been mixed.27, 28 AAML05P1 determined that KIR match can be determined prior to donor selection for pediatric patients with acute myelogenous leukemai (AML; n=90), but KIR mismatch does not improve survival, DFS, or relapse following in vivo T-cell depleted HCT.28
AAML1031:
Allogeneic HCT from the best available donor improves survival for patients with AML who have high-risk cytogenetics or mutation profiles, such as Fms-like tyrosine kinase (FLT3) mutations.29, 30 Among other research questions, AAML1031 tested whether adding sorafenib, a tyrosine kinase inhibitor (TKI) that inhibits constitutively activated FLT3 during induction and maintenance would improve outcomes for patients with high allelic ratio (AR >0.4) mutated FLT3. Patients who were not treated with sorafenib (n=76) had worse DFS (HR 2.28, p=0.032) than patients who were treated with sorafenib (n=72). Although more patients who received sorafenib underwent allogeneic HCT (64% vs 25%, p<0.001), the benefit of sorafenib was confirmed by multivariate analysis that accounted for HCT use and concurrent favorable co-mutations.31
AALL1331:
A CT-ALL collaboration standardized the HCT approach for ALL and showed that two cycles of blinatumomab improved survival and decreased toxicity compared to intensive chemotherapy prior to HCT for relapsed ALL, establishing a new standard of care.32 The survival advantage from blinatumomab was likely a result from more patients achieving a suitable remission status and receiving HCT. Future trials should test whether patients should proceed directly to HCT after 1 cycle of blinatumomab if they have achieved MRD negativity.
ANHL1522:
A CT-NHL collaboration evaluated “off the shelf” Epstein Barr virus (EBV) specific T cells for the treatment of post-transplant lymphoproliferative disease (PTLD) refractory to rituximab in pediatric solid organ transplant (SOT) recipients. The trial was closed early after 15 of a planned 30 patients enrolled due to slow accrual. Administering novel T-cell therapies in a cooperative group setting was feasible but too few patients were studied to assess efficacy (NCT02900976).
Collaborations with Cancer Control (Table 3):
Table 3:
Supportive Care Studies:
| First Author |
COG Study |
Reference | Findings | Outcome/Follow-up Study |
|---|---|---|---|---|
| Zerr | ACCL1034 | 59 | Chlorhexidine gluconate (CHG) cloth bathing (n=88) vs placebo (n=87) to reduce central venous catheter associated blood stream infection (CLABSI). Cumulative incidence of CLABSI was similar for CHG (35%) vs control (24%, p=0.09). CHG was associated with a higher risk of cutaneous Staph isolates resistant to CHG. | CHG baths not recommended for prevention of CLABSI |
| Alexander | ACCL0934 | 33 | Randomized pts with leukemia (AML or relapsed ALL, n=200) or HCT pts (n=424) to levofloxacin prophylaxis or none. Acute leukemia patients had less bacteremia if they received levofloxacin (22 vs 43%, p=0.001), but no significant difference in HCT patients (11% vs 17%, p=0.06) | Levofloxacin prophylaxis not recommended for prevention of bacteremia in HCT patients. |
| Analysis ongoing | ACCL1633 | Randomized trial of lactobacillus vs placebo to decrease incidence of GI acute GVHD was found to be futile at the first interim analysis. | Correlative studies of microbiome, biomarkers, and transplant outcomes underway. | |
| Dvorak | ACCL1131 | 34 | Patients undergoing allogeneic HCT were randomized to caspofungin (n=144) or triazole (fluconazole n=100, voriconazole n=46). Planned analysis found rates of IFD (1.4%) too low to detect differences between arms and study was closed early. | |
| Otto | ACCL1131 – Ancillary study | 35 | Weekly beta-D-glucan levels from 51 patients were correlated with IFD. None of the 25 patients with at least 1 positive test developed IFI (false positive rate 100%). | Serial monitoring of BDG for fungal surveillance not recommended. |
Infectious complications are common post-HCT due to prolonged neutropenia, mucosal barrier damage, and extended treatment with immunosuppressive drugs. Two recent COG trials attempted to decrease infectious complications, but unfortunately were unsuccessful. ACCL0934 randomized patients undergoing HCT to receive levofloxacin prophylaxis (n=210) or no prophylaxis (n=214) from two days prior to HCT until neutrophil recovery. Levofloxacin was not toxic, but bacteremia rates were not significantly different between the levofloxacin and no prophylaxis groups (11% vs 17%, p=0.06).33 ACCL1131 randomized patients to either caspofungin (n=144) or a triazole (n=146) as anti-fungal prophylaxis from the day of stem cell infusion until day 42 post-HCT or hospital discharge, whichever occurred first. The trial was powered to determine if caspofungin reduced the rate of proven or probable invasive fungal disease (IFD) by day 42 post-HCT from 7% to 2% compared to triazole . A planned interim analysis after 290 patients enrolled found very low rates of IFD in both cohorts (1.4% vs 1.4%, p=0.99), resulting in the study’s early closure.34 An embedded ancillary study evaluated serial beta-D-glucan (BDG) as a screening test for IFD in 51 patients. All 25 positive results were false positives, thus serial BDG monitoring is not a useful screening tool for IFD in pediatric HCT.35
ACCL1633:
Despite improvements in donor selection and GVHD prophylaxis strategies, grade II-IV acute GVHD occurs in approximately 40% of pediatric recipients of allogeneic HCT.36, 37 Several studies have linked changes in gastrointestinal (GI) microbial dysbiosis to transplant outcomes, including GVHD.38 Interventions that preserve or restore normal microbiota may reduce GI-associated toxicity and GVHD. ACCL1633 randomized HCT recipients to either oral Lactobacillus plantarum or placebo for 8 weeks. An interim analysis after the first 128 patients found no differences in the incidence of GI GVHD (unpublished data) and the study was closed for futility. Laboratory studies that utilize banked serial stool and blood samples to correlate the GI microbiome and serum biomarkers with clinical outcomes are currently ongoing.
Current Portfolio:
AALL1721:
CD19-CAR T cell therapy with tisagenlecleucel for relapsed and refractory B cell ALL dramatically improved survival rates from negligible with chemotherapy alone to 50-60% but many patients still do not experience benefit from this treatment.1, 39, 40 AALL1721, a CT-ALL collaboration tests the hypothesis that earlier treatment with tisagenlecleucel in high risk patients (i.e., MRD(+) at the end of consolidation) will produce higher rates of durable remission compared to other treatment strategies. The trial has enrolled 100 out of a planned 140 patients and is expected to complete accrual in 2023.
ASCT2031:
Allogeneic HCT is the best option for cure for many high-risk malignancies, but acute and chronic GVHD cause substantial short and long-term morbidity and mortality. Preliminary data from phase 2 studies in both children and adults showed that transplants from haploidentical donors using GVHD prophylaxis that included either T-cell receptor (TCR)αβ+ T cell/CD19+ B cell depletion or post-HCT cyclophosphamide with tacrolimus and mycophenolate prophylaxis (PTCy/Tac/MMF) reduced severe acute and chronic GVHD without increasing relapse or TRM.41, 42 ASCT2031 is a phase 3 randomized trial that hypothesizes that recipients of a haploidentical donor HCT using either TCRαβ+ T cell/CD19+ B cell depletion or PTCy/Tac/MMF prophylaxis will have less severe GVHD without more relapses than recipients of HLA-matched unrelated donor (MUD) HCT using standard tacrolimus and methotrexate prophylaxis. Eligible patients must have either acute leukemia or myelodysplastic syndrome (MDS) and access to both donor sources for randomization. Because certain racial and ethnic groups have a probability of <20% to identify a MUD,43 patients without access to both donor sources can be non-randomly assigned to a haploidentical arm. A study sub-aim will explore differences in outcomes by racial and ethnic group among patients who otherwise receive identical treatment. This trial opened in November 2022 and is expected to complete accrual in 4 years.
AAML1831 tests if a TKI more specific for FLT3, gilteritinib, improves outcomes in patients with AML with FLT3 abnormalities. High-risk patients with FLT3 alterations (based on a risk algorithm) are directed to allogeneic HCT from the best available donor followed by maintenance gilteritinib. The joint CT and AML task force designed the transplant portion of AAML1831 to reduce heterogeneity in transplant practice and simplify analyses. This trial is accruing about 230 patients per year.
Future Directions:
Cytomegalovirus (CMV) infection is common post-HCT, requires toxic treatment with antiviral drugs, and can be life-threatening.44 Letermovir is an antiviral medication approved by the U.S. Food and Drug Administration (FDA) for CMV prophylaxis in adult HCT recipients, but its efficacy in pediatric HCT is unknown.45 ACCL1932 will randomize pediatric allogeneic HCT recipients (2:1) to either 14 weeks of prophylaxis with letermovir or surveillance. The primary endpoint is the development of clinically significant CMV infection. The study will open in 2023.
ASCT2121 is an open label phase 2 study to test the efficacy low dose interleukin-2 (LD IL-2) as treatment for steroid refractory chronic GVHD (cGVHD) which develops in approximately 50% of HCT recipients and is the leading cause of late morbidity and mortality. Multiple single center adult and pediatric clinical trials with steroid-refractory cGVHD demonstrated efficacy for LD IL-2 with response rates as high as 80% in children and without any significant complications.46-48 COG and Cancer Therapy Evaluation Program (CTEP) approved a multicenter study to validate the efficacy of LD IL-2 using the same dose and schedule as previously published. The study will open after FDA approval and provided that drug supply can be obtained following the sale of IL-2 to a new supplier.
The AML/CT task force is a standing collaboration charged with developing new strategies to prevent relapse after HCT for AML. A small trial testing the combination of uproleselan, an E-selectin antagonist that sensitizes AML to chemotherapy, with intensive pre-HCT conditioning is currently underway through the PTCTC (NCT05569512) and may lead to a definitive COG trial, if warranted. Additionally, the task force is considering several possible post-HCT maintenance trial designs.
Improving the safety of allogeneic HCT remains a key priority, and GVHD remains the major driver for morbidity and TRM. Most cases of GVHD require treatment with systemic steroids that can cause numerous side effects, and children are uniquely susceptible to steroid-related impacts on growth and development.49 A multicenter study through the Mount Sinai Acute Graft versus Host Disease International Consortium (MAGIC) uses GVHD biomarkers to identify low risk GVHD patients at onset and then uses serial clinical and biomarker response monitoring to guide rapid steroid tapers. The goal of this study is to decrease toxicity and improve quality of life by reducing the cumulative exposure to systemic steroids (NCT05090384). MAGIC also studied steroid free treatment of GVHD using the JAK1 inhibitor itacitinib in adolescent and adult patients with biomarker-confirmed low-risk GVHD. Itacitinib monotherapy was as effective as systemic steroid treatment in a matched control group (response rate 89% vs 86%, p=0.67) and resulted in significantly fewer severe infections (27% vs 42%, p=0.04).50 Similar trials in younger pediatric cohorts could be facilitated through the COG CT committee.
Chronic and refractory viral infections remain a significant contributor to TRM following allogeneic HCT. Single or oligocenter trials of virus-specific T cells (VSTs) to treat these infections using expanded cells from seropositive donors, banks of VSTs from healthy donors, and genetically modified VSTs from either the donor or the patient have shown good response rates with limited toxicity.51 Building on ANHL1522, a future direction of the CT committee is to conduct definitive studies testing VSTs for infection control.
Engineered cellular therapy for B cell malignancies with CAR T cell therapy has changed the landscape of treatment of relapsed and refractory pediatric ALL. Future trials under development include a CT-ALL collaboration to test anti-CD19 CAR T-cells as primary therapy for ALL at first relapse. New immune effectors such as tumor specific engineered T-cells and engineered NK cells against a variety of oncologic targets including solid tumors are also actively being developed for future study within COG.
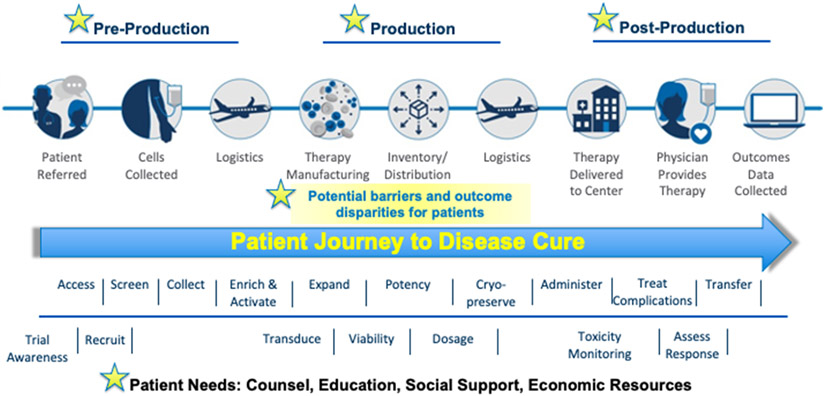
Cellular therapies for cancer and infection control face several challenges. First, these therapies are high risk and require complex orchestration of manufacturing and distribution (Figure 2). Second, compliance with Foundation for the Accreditation of Cellular Therapy (FACT) standards for immune effector cell therapies require substantial medical and administrative effort. Third, while the development of novel cellular therapies often occurs at individual sites, eligible patients are rare and studies to assess efficacy for patients with the greatest need due to poor prognosis with current treatment options (e.g., relapsed brain cancer, sarcomas) are not feasible for single centers. COG has an outstanding record of collaboration between diseases and disciplines to develop novel chemotherapy-based treatment trials, and this same level of engagement is needed to achieve the sample sizes needed for trials testing cellular therapies. COG AALL1721 demonstrated the ability to recruit patients, collect lymphocytes, ship to a central location for manufacturing, and return the cellular therapy product for infusion at the treating institution. Future trials testing innovative cellular therapies will benefit from pairing the expertise of the Developmental Therapeutics Committee in early phase clinical trials with the expertise of the CT committee in cell collection techniques and monitoring the unique toxicities associated with these therapies while testing efficacy.
Figure 2:
Intricate logistics involved in delivery of novel cellular therapy, including potential patient barriers. Adapted from ASTCT-NMDP ACCESS Initiative presentation, provided by Jeffrey Auletta.
Conclusions:
The COG CT committee conducts innovative trials to improve patient outcomes following autologous, allogeneic and immune effector cell therapies. Relapse remains a significant cause of treatment failure, and the CT committee strives to make further improvements through innovative trials of new preparative regimen strategies, incorporation of maintenance therapy, and by facilitating multicenter studies of relapsed or refractory solid tumors with emerging cellular treatments such as tumor specific lymphocytes52 or NK cell-based therapies53. Supportive care for CT patients is also critical, particularly for viral infections. A trial of CMV directed prophylaxis will open within the year. In addition, COG has existing infrastructure and CT expertise to explore multi-center trials evaluating antigen specific T cells targeting viruses,51 tumor-associated antigens,54, 55 and to control viral infections and malignancy in HCT patients. Finally, the broadening applicability of CAR-T cells targeting CD19+/−CD22 and other combination targets in the pediatric setting56 represents a unique opportunity for the COG CT committee to support advanced phased clinical trials for CAR-T approaches.
Table 4:
Abbreviations
| Abbreviation | Term |
|---|---|
| ALL | Acute lymphoblastic leukemia |
| AML | Acute myelogenous leukemia |
| AR | Allelic ratio |
| AT/RT | Atypical teratoid/rhabdoid tumor |
| AYA | Adolescent and young adult |
| BDG | Beta-D-glucan |
| BMT CTN | Blood and Marrow Clinical Trials Network |
| Bu-Cy-Mel | Busulfan-cyclophosphamide-melphalan |
| Bu-Flu | Busulfan-fludarabine |
| CAR-T | Chimeric antigen receptor T cell |
| CCL | Cancer Control |
| CEM | Carboplatin-etoposide-melphalan |
| cGVHD | Chronic GVHD |
| CMV | Cytomegalovirus |
| CNS | Central nervous system |
| COG | Children’s Oncology Group |
| CT | Cellular Therapy and Stem Cell Transplantation Committee |
| CTEP | Cancer Therapy Evaluation Program |
| DFS | Disease free survival |
| EBV | Epstein Barr virus |
| EFS | Event free survival |
| FACT | Foundation for the Accreditation of Cellular Therapy |
| FDA | U.S. Food and Drug Administration |
| FLT3 | Fms-like tyrosine kinase |
| GI | Gastrointestinal |
| GVHD | Graft-versus-Host disease |
| GVL | Graft-versus-leukemia |
| HCT | Hematopoietic cell transplantion |
| HLA | Human leukocyte antigen |
| HR | Hazard ratio |
| IFD | Invasive fungal disease |
| IgH | Immunoglobulin heavy chain |
| JMML | Juvenile myelomonocytic leukemia |
| KIR | Killer immunoglobulin receptor |
| LD IL-2 | Low dose interleukin-2 |
| MAGIC | Mount Sinai Acute Graft versus Host Disease International Consortium |
| MDS | Myelodysplastic syndrome |
| MRD | Minimal residual disease |
| MUD | Matched unrelated donor |
| NB | Neuroblastoma |
| NGS | Next-generation sequencing |
| OS | Overall survival |
| PTCTC | Pediatric Transplant and Cellular Therapy Consortium |
| PTCy/Tac/MMF | Post-HCT cyclophosphamide with tacrolimus and mycophenolate |
| PTLD | Post-transplant lymphoproliferative disorder |
| SOT | Solid organ transplant |
| TBI | Total body irradiation |
| TCR | T-cell receptor |
| TKI | Tyrosine kinase inhibitor |
| TRM | Transplant related mortality |
| UCB | Umbilical cord blood |
| V[D]J | Variable, diversity, and joining |
| VOD/SOS | Veno-occlusive disease/sinusoidal obstructive syndrome |
| VSTs | Virus-specific T-cells |
| 131I-MIBG | 131I-meta-iodobenzylguanidine |
Funding support:
Grant support from the National Institute of Health, U10CA180886, U10CA180899, U10CA098543, U10CA098413.
Footnotes
Conflict of interest disclosure: JEL has a patent for GVHD biomarkers and receives royalties from Viracor. CLK was on an advisory board for Horizon Therapeutics.
References:
- 1.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med. Feb 1 2018;378(5):439–448. doi: 10.1056/NEJMoa1709866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frangoul H, Altshuler D, Cappellini MD, et al. CRISPR-Cas9 Gene Editing for Sickle Cell Disease and beta-Thalassemia. N Engl J Med. Jan 21 2021;384(3):252–260. doi: 10.1056/NEJMoa2031054 [DOI] [PubMed] [Google Scholar]
- 3.Locatelli F, Thompson AA, Kwiatkowski JL, et al. Betibeglogene Autotemcel Gene Therapy for Non-beta(0)/beta(0) Genotype beta-Thalassemia. N Engl J Med. Feb 3 2022;386(5):415–427. doi: 10.1056/NEJMoa2113206 [DOI] [PubMed] [Google Scholar]
- 4.Pulsipher MA, Langholz B, Wall DA, et al. The addition of sirolimus to tacrolimus/methotrexate GVHD prophylaxis in children with ALL: a phase 3 Children's Oncology Group/Pediatric Blood and Marrow Transplant Consortium trial. Blood. Mar 27 2014;123(13):2017–25. doi: 10.1182/blood-2013-10-534297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pulsipher MA, Langholz B, Wall DA, et al. Risk factors and timing of relapse after allogeneic transplantation in pediatric ALL: for whom and when should interventions be tested? Bone Marrow Transplant. Sep 2015;50(9):1173–9. doi: 10.1038/bmt.2015.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bader P, Salzmann-Manrique E, Balduzzi A, et al. More precisely defining risk peri-HCT in pediatric ALL: pre- vs post-MRD measures, serial positivity, and risk modeling. Blood Adv. Nov 12 2019;3(21):3393–3405. doi: 10.1182/bloodadvances.2019000449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pulsipher MA, Carlson C, Langholz B, et al. IgH-V(D)J NGS-MRD measurement pre- and early post-allotransplant defines very low- and very high-risk ALL patients. Blood. May 28 2015;125(22):3501–8. doi: 10.1182/blood-2014-12-615757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peters C, Dalle JH, Locatelli F, et al. Total Body Irradiation or Chemotherapy Conditioning in Childhood ALL: A Multinational, Randomized, Noninferiority Phase III Study. J Clin Oncol. Feb 1 2021;39(4):295–307. doi: 10.1200/JCO.20.02529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bunin N, Aplenc R, Kamani N, Shaw K, Cnaan A, Simms S. Randomized trial of busulfan vs total body irradiation containing conditioning regimens for children with acute lymphoblastic leukemia: a Pediatric Blood and Marrow Transplant Consortium study. Bone Marrow Transplant. Sep 2003;32(6):543–8. doi: 10.1038/sj.bmt.1704198 [DOI] [PubMed] [Google Scholar]
- 10.Gupta V, Braun TM, Chowdhury M, Tewari M, Choi SW. A Systematic Review of Machine Learning Techniques in Hematopoietic Stem Cell Transplantation (HSCT). Sensors (Basel). Oct 27 2020;20(21)doi: 10.3390/s20216100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Locatelli F, Crotta A, Ruggeri A, et al. Analysis of risk factors influencing outcomes after cord blood transplantation in children with juvenile myelomonocytic leukemia: a EUROCORD, EBMT, EWOG-MDS, CIBMTR study. Blood. Sep 19 2013;122(12):2135–41. doi: 10.1182/blood-2013-03-491589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yabe M, Ohtsuka Y, Watanabe K, et al. Transplantation for juvenile myelomonocytic leukemia: a retrospective study of 30 children treated with a regimen of busulfan, fludarabine, and melphalan. Int J Hematol. Feb 2015;101(2):184–90. doi: 10.1007/s12185-014-1715-7 [DOI] [PubMed] [Google Scholar]
- 13.Dvorak CC, Satwani P, Stieglitz E, et al. Disease burden and conditioning regimens in ASCT1221, a randomized phase II trial in children with juvenile myelomonocytic leukemia: A Children's Oncology Group study. Pediatr Blood Cancer. Jul 2018;65(7):e27034. doi: 10.1002/pbc.27034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurtzberg J, Prasad VK, Carter SL, et al. Results of the Cord Blood Transplantation Study (COBLT): clinical outcomes of unrelated donor umbilical cord blood transplantation in pediatric patients with hematologic malignancies. Blood. Nov 15 2008;112(10):4318–27. doi: 10.1182/blood-2007-06-098020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cornetta K, Laughlin M, Carter S, et al. Umbilical cord blood transplantation in adults: results of the prospective Cord Blood Transplantation (COBLT). Biol Blood Marrow Transplant. Feb 2005;11(2):149–60. doi: 10.1016/j.bbmt.2004.11.020 [DOI] [PubMed] [Google Scholar]
- 16.Brunstein CG, Barker JN, Weisdorf DJ, et al. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplantation outcomes in 110 adults with hematologic disease. Blood. Oct 15 2007;110(8):3064–70. doi: 10.1182/blood-2007-04-067215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wagner JE Jr., Eapen M, Carter S, et al. One-unit versus two-unit cord-blood transplantation for hematologic cancers. N Engl J Med. Oct 30 2014;371(18):1685–94. doi: 10.1056/NEJMoa1405584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kreissman SG, Seeger RC, Matthay KK, et al. Purged versus non-purged peripheral blood stem-cell transplantation for high-risk neuroblastoma (COG A3973): a randomised phase 3 trial. Lancet Oncol. Sep 2013;14(10):999–1008. doi: 10.1016/S1470-2045(13)70309-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seif AE, Naranjo A, Baker DL, et al. A pilot study of tandem high-dose chemotherapy with stem cell rescue as consolidation for high-risk neuroblastoma: Children's Oncology Group study ANBL00P1. Bone Marrow Transplant. Jul 2013;48(7):947–52. doi: 10.1038/bmt.2012.276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grupp SA, Stern JW, Bunin N, et al. Tandem high-dose therapy in rapid sequence for children with high-risk neuroblastoma. J Clin Oncol. Jul 2000;18(13):2567–75. doi: 10.1200/JCO.2000.18.13.2567 [DOI] [PubMed] [Google Scholar]
- 21.Park JR, Kreissman SG, London WB, et al. Effect of Tandem Autologous Stem Cell Transplant vs Single Transplant on Event-Free Survival in Patients With High-Risk Neuroblastoma: A Randomized Clinical Trial. JAMA. Aug 27 2019;322(8):746–755. doi: 10.1001/jama.2019.11642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ladenstein R, Potschger U, Pearson ADJ, et al. Busulfan and melphalan versus carboplatin, etoposide, and melphalan as high-dose chemotherapy for high-risk neuroblastoma (HR-NBL1/SIOPEN): an international, randomised, multi-arm, open-label, phase 3 trial. Lancet Oncol. Apr 2017;18(4):500–514. doi: 10.1016/S1470-2045(17)30070-0 [DOI] [PubMed] [Google Scholar]
- 23.Granger MM, Naranjo A, Bagatell R, et al. Myeloablative Busulfan/Melphalan Consolidation following Induction Chemotherapy for Patients with Newly Diagnosed High-Risk Neuroblastoma: Children's Oncology Group Trial ANBL12P1. Transplant Cell Ther. Jun 2021;27(6):490 e1–490 e8. doi: 10.1016/j.jtct.2021.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reddy AT, Strother DR, Judkins AR, et al. Efficacy of High-Dose Chemotherapy and Three-Dimensional Conformal Radiation for Atypical Teratoid/Rhabdoid Tumor: A Report From the Children's Oncology Group Trial ACNS0333. J Clin Oncol. Apr 10 2020;38(11):1175–1185. doi: 10.1200/JCO.19.01776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rare Tumors in Kids May Respond to Tazemetostat. Cancer Discov. Jan 2018;8(1):OF5. doi: 10.1158/2159-8290.CD-NB2017-152 [DOI] [PubMed] [Google Scholar]
- 26.Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. Mar 15 2002;295(5562):2097–100. doi: 10.1126/science.1068440 [DOI] [PubMed] [Google Scholar]
- 27.Hsu KC, Keever-Taylor CA, Wilton A, et al. Improved outcome in HLA-identical sibling hematopoietic stem-cell transplantation for acute myelogenous leukemia predicted by KIR and HLA genotypes. Blood. Jun 15 2005;105(12):4878–84. doi: 10.1182/blood-2004-12-4825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davies SM, Ruggieri L, DeFor T, et al. Evaluation of KIR ligand incompatibility in mismatched unrelated donor hematopoietic transplants. Killer immunoglobulin-like receptor. Blood. Nov 15 2002;100(10):3825–7. doi: 10.1182/blood-2002-04-1197 [DOI] [PubMed] [Google Scholar]
- 29.Horan JT, Alonzo TA, Lyman GH, et al. Impact of disease risk on efficacy of matched related bone marrow transplantation for pediatric acute myeloid leukemia: the Children's Oncology Group. J Clin Oncol. Dec 10 2008;26(35):5797–801. doi: 10.1200/JCO.2007.13.5244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Masetti R, Muratore E, Gori D, Prete A, Locatelli F. Allogeneic hematopoietic stem cell transplantation for pediatric acute myeloid leukemia in first complete remission: a meta-analysis. Ann Hematol. Nov 2022;101(11):2497–2506. doi: 10.1007/s00277-022-04965-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pollard JA, Alonzo TA, Gerbing R, et al. Sorafenib in Combination With Standard Chemotherapy for Children With High Allelic Ratio FLT3/ITD+ Acute Myeloid Leukemia: A Report From the Children's Oncology Group Protocol AAML1031. J Clin Oncol. Jun 20 2022;40(18):2023–2035. doi: 10.1200/JCO.21.01612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown PA, Ji L, Xu X, et al. Effect of Postreinduction Therapy Consolidation With Blinatumomab vs Chemotherapy on Disease-Free Survival in Children, Adolescents, and Young Adults With First Relapse of B-Cell Acute Lymphoblastic Leukemia: A Randomized Clinical Trial. JAMA. Mar 2 2021;325(9):833–842. doi: 10.1001/jama.2021.0669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alexander S, Fisher BT, Gaur AH, et al. Effect of Levofloxacin Prophylaxis on Bacteremia in Children With Acute Leukemia or Undergoing Hematopoietic Stem Cell Transplantation: A Randomized Clinical Trial. JAMA. Sep 11 2018;320(10):995–1004. doi: 10.1001/jama.2018.12512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dvorak CC, Fisher BT, Esbenshade AJ, et al. A Randomized Trial of Caspofungin vs Triazoles Prophylaxis for Invasive Fungal Disease in Pediatric Allogeneic Hematopoietic Cell Transplant. J Pediatric Infect Dis Soc. Apr 30 2021;10(4):417–425. doi: 10.1093/jpids/piaa119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Otto WR, Dvorak CC, Boge CLK, et al. Prospective Evaluation of the Fungitell(R) (1-->3) Beta-D-Glucan Assay as a Diagnostic Tool for Invasive Fungal Disease in Pediatric Allogeneic Hematopoietic Cell Transplantation: A Report from the Children's Oncology Group. Pediatr Transplant. Feb 2023;27(1):e14399. doi: 10.1111/petr.14399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andolina JR, Wang YC, Ji L, et al. Adolescent and young adult (AYA) versus pediatric patients with acute leukemia have a significantly increased risk of acute GVHD following unrelated donor (URD) stem cell transplantation (SCT): the Children's Oncology Group experience. Bone Marrow Transplant. Mar 2022;57(3):445–452. doi: 10.1038/s41409-021-01558-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qayed M, Wang T, Hemmer MT, et al. Influence of Age on Acute and Chronic GVHD in Children Undergoing HLA-Identical Sibling Bone Marrow Transplantation for Acute Leukemia: Implications for Prophylaxis. Biol Blood Marrow Transplant. Mar 2018;24(3):521–528. doi: 10.1016/j.bbmt.2017.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nguyen CL, Docampo MD, van den Brink MR, Markey KA. The role of the intestinal microbiota in allogeneic HCT: clinical associations and preclinical mechanisms. Curr Opin Genet Dev. Feb 2021;66:25–35. doi: 10.1016/j.gde.2020.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. Oct 16 2014;371(16):1507–17. doi: 10.1056/NEJMoa1407222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schultz LM, Baggott C, Prabhu S, et al. Disease Burden Affects Outcomes in Pediatric and Young Adult B-Cell Lymphoblastic Leukemia After Commercial Tisagenlecleucel: A Pediatric Real-World Chimeric Antigen Receptor Consortium Report. J Clin Oncol. Mar 20 2022;40(9):945–955. doi: 10.1200/JCO.20.03585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Symons HJ, Zahurak M, Cao Y, et al. Myeloablative haploidentical BMT with posttransplant cyclophosphamide for hematologic malignancies in children and adults. Blood Adv. Aug 25 2020;4(16):3913–3925. doi: 10.1182/bloodadvances.2020001648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Merli P, Pagliara D, Mina T, et al. alphabetaT- and B-cell-depleted HLA-haploidentical hematopoietic stem cell transplantation in children with myelodysplastic syndromes. Haematologica. Dec 1 2022;107(12):2966–2971. doi: 10.3324/haematol.2022.280698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gragert L, Eapen M, Williams E, et al. HLA match likelihoods for hematopoietic stem-cell grafts in the U.S. registry. N Engl J Med. Jul 24 2014;371(4):339–48. doi: 10.1056/NEJMsa1311707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Teira P, Battiwalla M, Ramanathan M, et al. Early cytomegalovirus reactivation remains associated with increased transplant-related mortality in the current era: a CIBMTR analysis. Blood. May 19 2016;127(20):2427–38. doi: 10.1182/blood-2015-11-679639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marty FM, Ljungman P, Chemaly RF, et al. Letermovir prophylaxis for cytomegalovirus in hematopoietic-cell transplantation. N Engl J Med. Dec 21 2017;377(25):2433–2444. doi: 10.1056/NEJMoa1706640 [DOI] [PubMed] [Google Scholar]
- 46.Whangbo JS, Kim HT, Mirkovic N, et al. Dose-escalated interleukin-2 therapy for refractory chronic graft-versus-host disease in adults and children. Blood Adv. Sep 10 2019;3(17):2550–2561. doi: 10.1182/bloodadvances.2019000631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koreth J, Kim HT, Jones KT, et al. Efficacy, durability, and response predictors of low-dose interleukin-2 therapy for chronic graft-versus-host disease. Blood. Jul 7 2016;128(1):130–7. doi: 10.1182/blood-2016-02-702852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koreth J, Matsuoka K, Kim HT, et al. Interleukin-2 and regulatory T cells in graft-versus-host disease. N Engl J Med. Dec 1 2011;365(22):2055–66. doi: 10.1056/NEJMoa1108188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aljebab F, Choonara I, Conroy S. Systematic Review of the Toxicity of Long-Course Oral Corticosteroids in Children. PLoS One. 2017;12(1):e0170259. doi: 10.1371/journal.pone.0170259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Etra A, Capellini A, Alousi A, et al. Effective treatment of low-risk acute GVHD with itacitinib monotherapy. Blood. Feb 2 2023;141(5):481–489. doi: 10.1182/blood.2022017442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Motta CM, Keller MD, Bollard CM. Applications of virus-specific T cell therapies post-BMT. Semin Hematol. Jan 2023;60(1):10–19. doi: 10.1053/j.seminhematol.2022.12.002 [DOI] [PubMed] [Google Scholar]
- 52.Hont AB, Cruz CR, Ulrey R, et al. Immunotherapy of Relapsed and Refractory Solid Tumors With Ex Vivo Expanded Multi-Tumor Associated Antigen Specific Cytotoxic T Lymphocytes: A Phase I Study. J Clin Oncol. Sep 10 2019;37(26):2349–2359. doi: 10.1200/JCO.19.00177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Elmas E, Saljoughian N, de Souza Fernandes Pereira M, et al. CRISPR Gene Editing of Human Primary NK and T Cells for Cancer Immunotherapy. Front Oncol. 2022;12:834002. doi: 10.3389/fonc.2022.834002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kinoshita H, Cooke KR, Grant M, et al. Outcome of donor-derived TAA-T cell therapy in patients with high-risk or relapsed acute leukemia post allogeneic BMT. Blood Adv. Apr 26 2022;6(8):2520–2534. doi: 10.1182/bloodadvances.2021006831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Naik S, Vasileiou S, Tzannou I, et al. Donor-derived multiple leukemia antigen-specific T-cell therapy to prevent relapse after transplant in patients with ALL. Blood. Apr 28 2022;139(17):2706–2711. doi: 10.1182/blood.2021014648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shalabi H, Qin H, Su A, et al. CD19/22 CAR T cells in children and young adults with B-ALL: phase 1 results and development of a novel bicistronic CAR. Blood. Aug 4 2022;140(5):451–463. doi: 10.1182/blood.2022015795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yanik GA, Mineishi S, Levine JE, et al. Soluble tumor necrosis factor receptor: enbrel (etanercept) for subacute pulmonary dysfunction following allogeneic stem cell transplantation. Biol Blood Marrow Transplant. Jul 2012;18(7):1044–54. doi: 10.1016/j.bbmt.2011.11.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schofield HT, Fabrizio VA, Braniecki S, et al. Monitoring Neurocognitive Functioning After Pediatric Cellular Therapy or Hematopoietic Cell Transplant: Guidelines From the COG Neurocognition in Cellular Therapies Task Force. Transplant Cell Ther. Oct 2022;28(10):625–636. doi: 10.1016/j.jtct.2022.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zerr DM, Milstone AM, Dvorak CC, et al. Chlorhexidine gluconate bathing in children with cancer or those undergoing hematopoietic stem cell transplantation: A double-blinded randomized controlled trial from the Children's Oncology Group. Cancer. Jan 1 2021;127(1):56–66. doi: 10.1002/cncr.33271 [DOI] [PMC free article] [PubMed] [Google Scholar]