Figure 2.
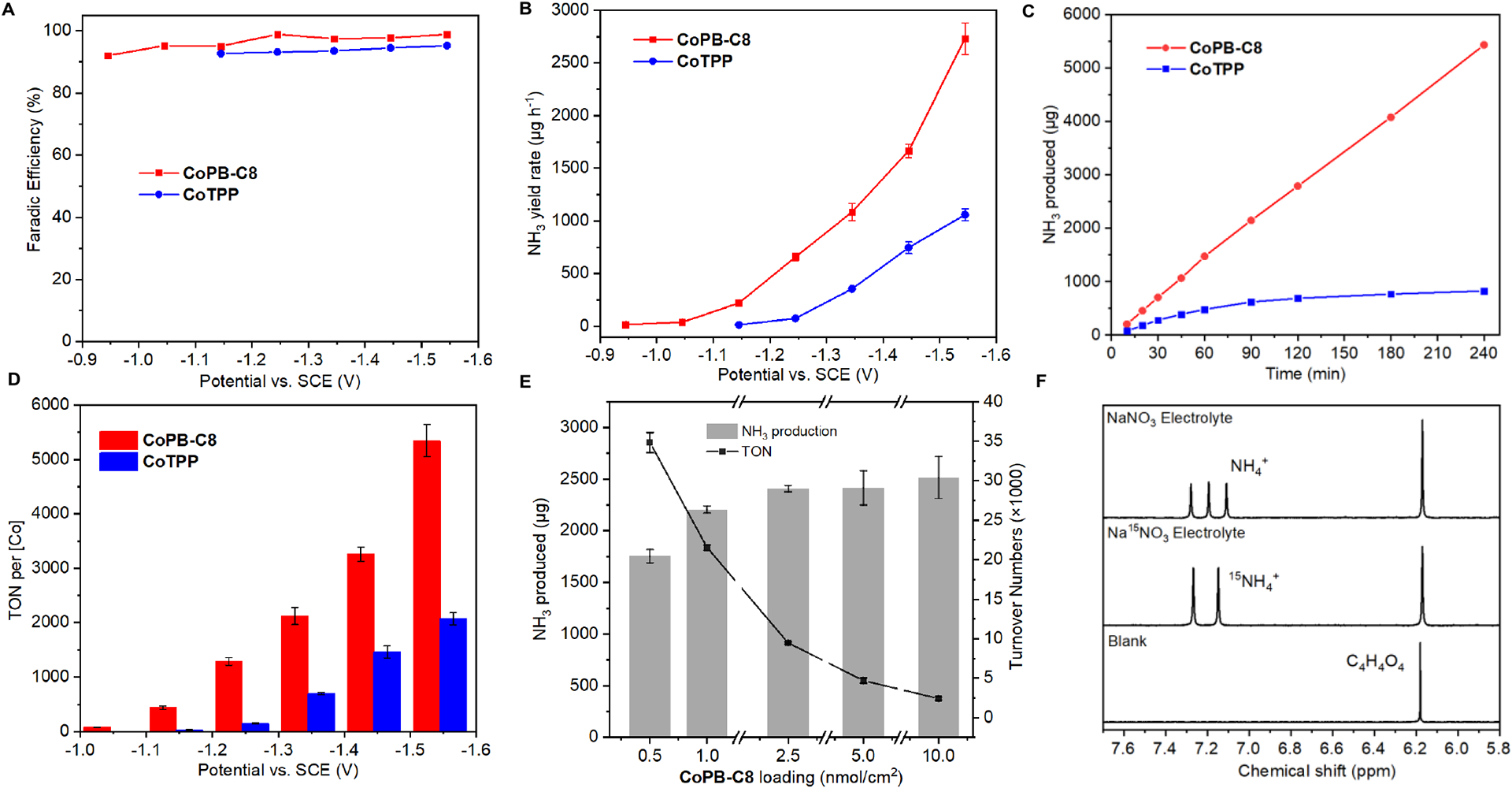
Supramolecular cobalt porphyrin box organic cage shows superior electrochemical nitrate reduction (NO3RR) activity relative to a parent cobalt tetraphenylporphyrin control analog. (A) Faradaic efficiency (FE) for electrochemical nitrate reduction to ammonia, plotted by NH3 production, catalyzed by CoPB-C8 (red) and CoTPP (blue) over a range of applied potentials after 1 h electrolysis. (B) NH3 yield rate for CoPB-C8 (red), and CoTPP (blue). (C) NH3 production during 4 h electrolysis at −1.34 V vs. SCE for CoPB-C8 (red), and CoTPP (blue). (D) Comparison of turnover number (TON) values per Co over a range of applied potentials after 1 h electrolysis. (E) NH3 production and TON for various amounts of CoPB-C8 loading at −1.54 V vs. SCE. (F) 1H NMR spectra showing direct detection of NH3 product with 15N labeling of substrate for electrolysis runs, after three independent nitrate reduction tests at −1.44 V vs SCE, 0.5 M Na2SO4/0.1 M NaNO3, 0.5 M Na2SO4/0.1 M Na15NO3, and 0.5 M Na2SO4. Maleic acid (C4H4O4) was added as an internal standard.
