Figure 3. Molecular mechanisms of dietary restriction.
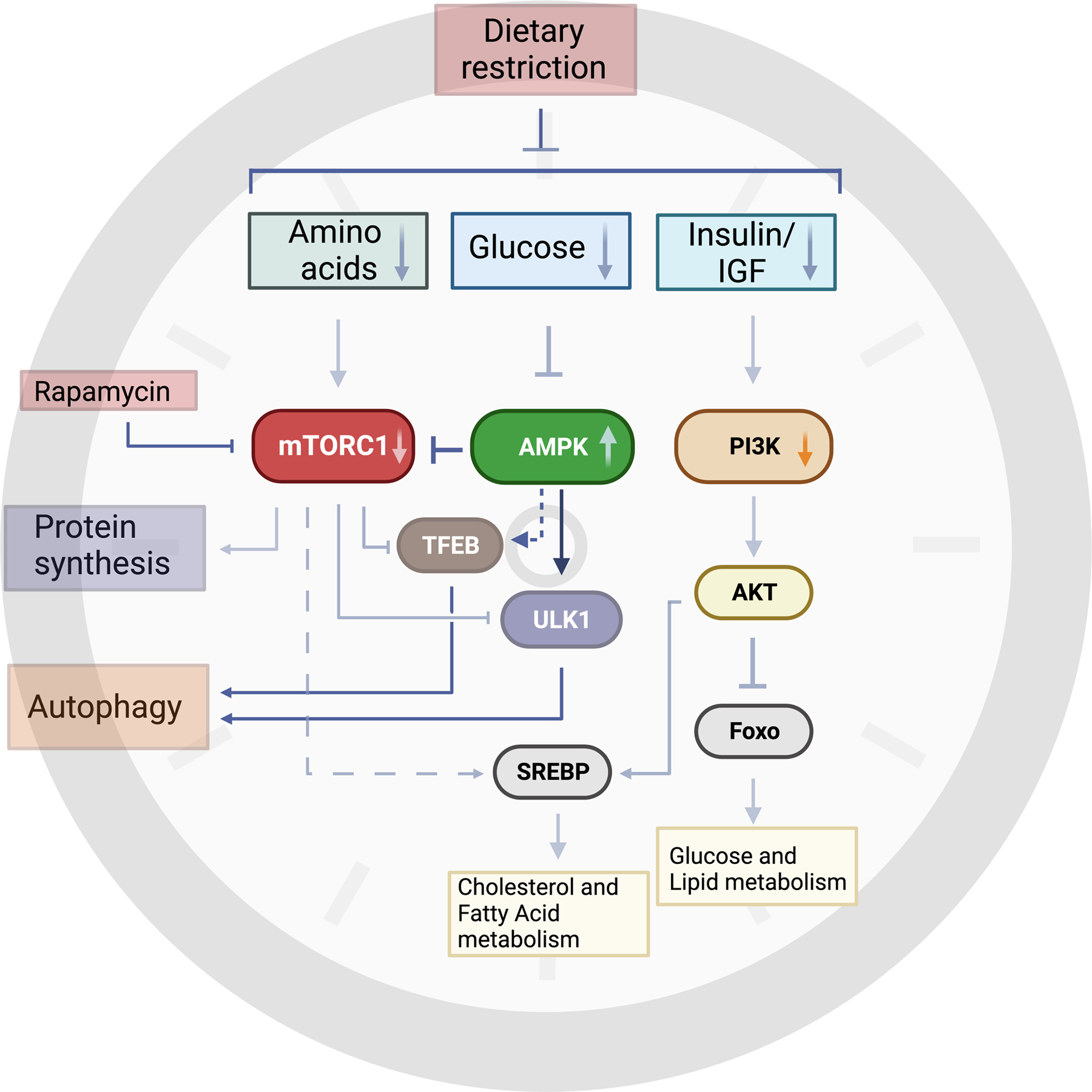
Dietary restriction encompasses reduced consumption of macronutrients such as carbohydrates and amino acids to alter their blood levels and, subsequently, insulin and IGF levels. These changes are sensed across different cell types and impinge on several conserved nutrient sensors, such as mTOR complex 1 (mTORC1) and AMP- activated protein kinase (AMPK). Reduced mTORC1 activity, due to lower levels of certain amino acids, leads to decreased protein synthesis and ribosomal biogenesis. AMPK acts as a sensor of cellular energy by sensing changes in intracellular AMP, ADP and ATP levels. Glucose deprivation activates AMPK which in turn can phosphorylate and regulate several downstream substrates. AMPK and mTORC1 both converge on regulating autophagy in opposing ways. AMPK-dependent phosphorylation of Unc-51-like kinase 1 (ULK1) is required for mitophagy, a specific type of autophagy that involves degrading damaged mitochondria that may be impaired in aged tissues. Dietary restriction of carbohydrates or overall calorie restriction can also reduce metabolic activity through PI3K and AKT pathways. Reduced AKT activity will increase forkhead box protein O (Foxo)-dependent transcriptional programs involved in glucose and lipid signaling. Additionally, mTORC1 and AKT regulate sterol regulatory element-binding protein (SREBP) 1 and 2, which regulate fatty acid and cholesterol metabolism, respectively. Inhibition of mTORC1 through Rapamycin can mimic some of the beneficial effects of dietary restriction, but it remains to be seen if Rapamycin affects different tissues and cell types to the same extent. This figure was created with BioRender.com.
