Fig. 8. Cells under chronic allostatic load display accelerated cellular aging.
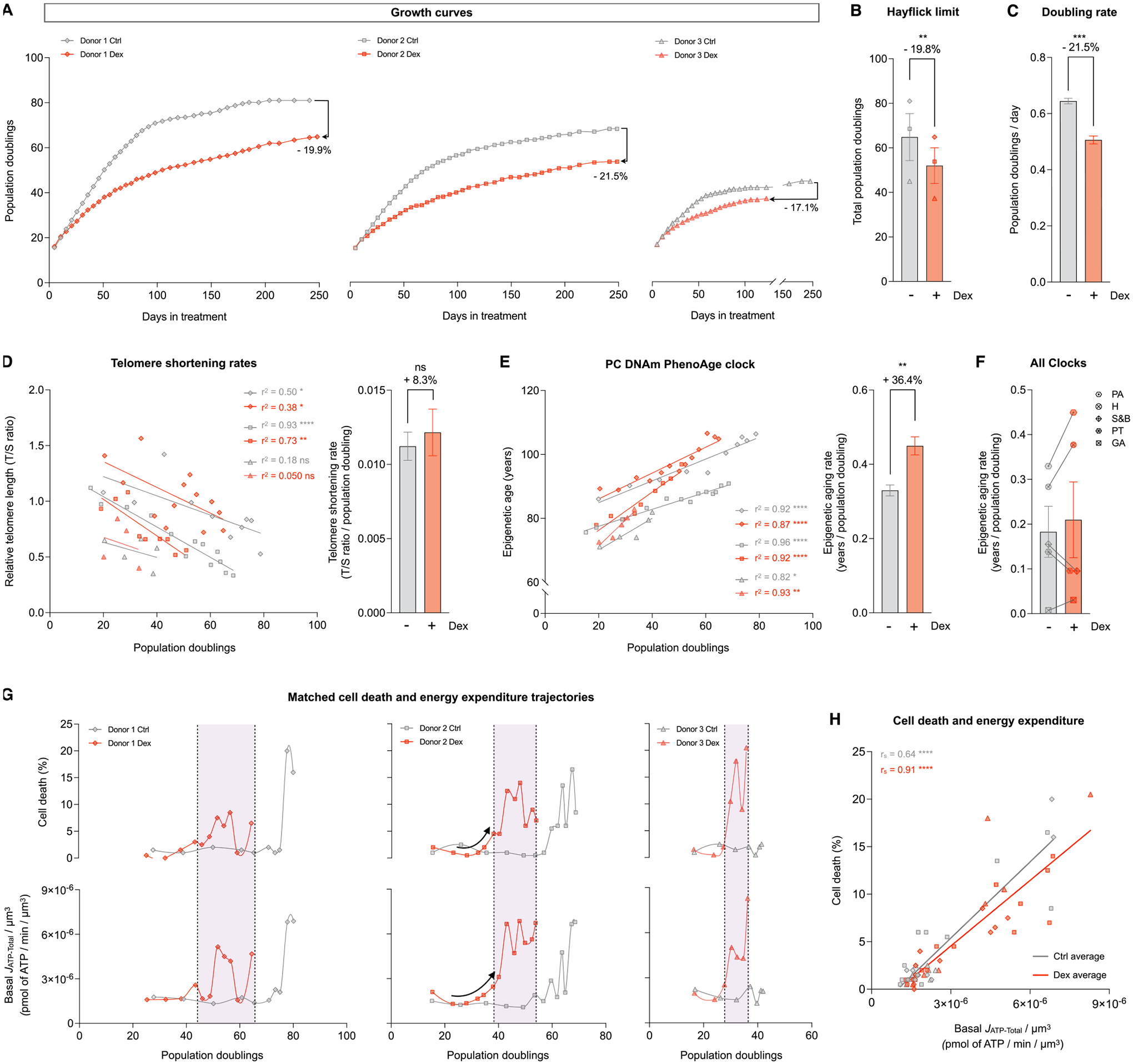
(A) Lifespan trajectories of cumulative population doublings. (B) Hayflick limit for each donor of each group. (C) Group average early life doubling rate, inferred by linear mixed model of the population doubling trajectories within the first 50 days of treatment. (D) Telomere length across population doublings, with simple linear regressions for each donor of each group (left panel), and group average telomere shortening rate inferred by linear mixed model along the whole lifespan (right panel). (E) Epigenetic age calculated by the principal components (PC) PhenoAge epigenetic clock, with linear regressions for each donor of each group (left panel), and group average epigenetic aging rate inferred by linear mixed model along the whole lifespan (right panel). (F) Epigenetic aging rate for all the PC epigenetic clocks evaluated: PA: PhenoAge, H: Hannum, S&B: Skin and Blood, PT: PanTissue, GA: GrimAge. Detailed analysis of these epigenetic clocks is in Supplementary Material Fig. S11 (G) Percentage of dead cells (upper panels) and basal JATP-Total/cell volume (lower panels) across population doublings for Donor 1 (left panels), Donor 2 (middle panels) and Donor 3 (right panels). (H) Correlation between proportion of dead cells in every passage and Basal JATP-Total/cell volume. n = 3 donors per group; timepoints per donor: n = 26–36 in A, n = 4–14 in D-E, n = 8–13 in G-H. Bar graphs are mean ± SEM, Satterthwaite method. Correlation graphs show Spearman r and thick lines represent simple linear regression for each group. * p < 0.05, * * p < 0.01, * ** * p < 0.0001, ns: not significant.
