Figure 1. Microglial responses in Alzheimer’s Disease.
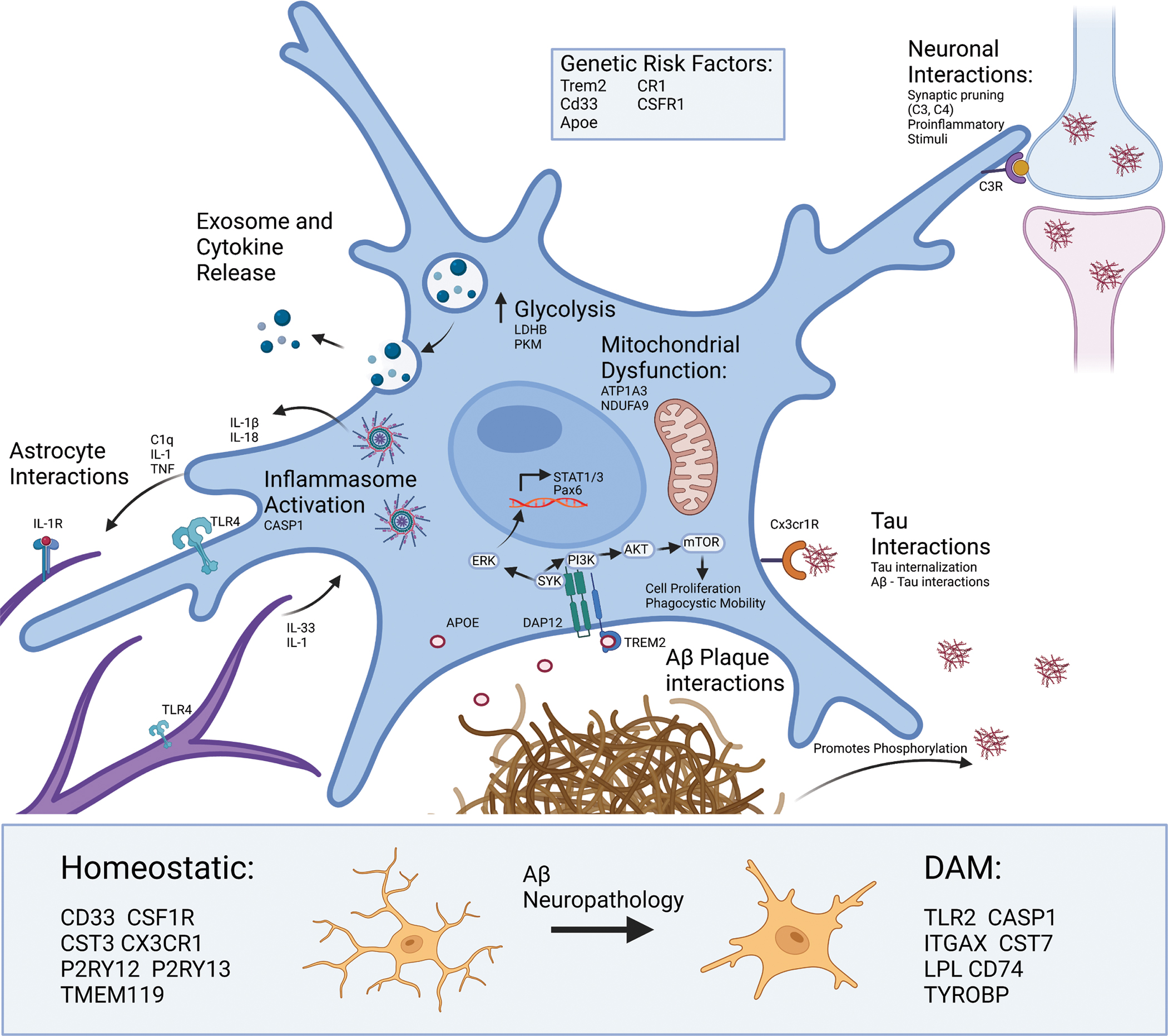
Several genetic risk loci for AD (TREM2, CD33, CSF1R, CR1, APOE) encode proteins involved in signaling cascades that support broad phenotypic shifts in motility, metabolism, phagocytosis, proliferation, cytokine production, exosome production and release, and apoptosis. Microglia use complement signaling to identify both healthy and dead neurons for degradation. Microglia can use signaling cascades to detect and recruit to pathological proteins including TAU and Aβ. For example, fractalkine signaling (via fractalkine receptor CX3CR1) allows microglia to detect and phagocytose Tau [54,216,217]. Microglial interactions with Aβ plaques are distinct, where microglia actively surround Aβ plaques and interact with them via mechanisms involving APOE, TREM2 and its receptor. This receptor is responsible for the TREM2-dependent signaling pathway which results in activation of SYK and ERK [218,219]. ERK then crosses the nucleus to allow for transcription of key inflammatory signaling molecules such as STAT1. Whereas activation of the mTOR pathway leads to further phagocytosis and cell proliferation. TAU and Aβ can also directly interact with each other, where tau tangles can act as a seed for Aβ plaque accumulation and Aβ can promote the phosphorylation of tau necessary for tau fibrillization. Cross-talk between microglia and astrocytes is largely responsible for activation of the TLR4 and IL1R on both cell types which can shift astrocytes and microglia toward more proinflammatory cytokine release [77]. Proteomic studies evidence an increase in CASP1 as well as IL-1β indicating formation and activation of the NLRP3 inflammasome which cleaves proIL-1β and proIL-18 prior to release from microglia. These signaling events also result in increased production and release of exosomes that contain key signaling proteins like TNF, and other cargo. This corresponds with metabolic reprograming in microglia, where there is an increased movement of glucose to glycolysis but a reduction in mitochondrial activity, which is in turn sustains ATP production and calcium-dependent mechanisms necessary for DAM function. Aβ and other neuropathologies also transform microglia from homeostatic and disease-associated microglia (DAM) states, and key markers of these states also shown below. [16,220]
