Abstract
Objective:
Subphenotypes of asthma may be determined by age onset and atopic status. We sought to characterize early or late onset atopic asthma with fungal or non-fungal sensitization (AAFS or AANFS) and non-atopic asthma (NAA) in children and adults in the Severe Asthma Research Program (SARP). SARP is an ongoing project involving well-phenotyped patients with mild to severe asthma.
Methods:
Phenotypic comparisons were performed using Kruskal-Wallis or chi-square test. Genetic association analyses were performed using logistic or linear regression.
Results:
Airway hyper-responsiveness, total serum IgE levels, and T2 biomarkers showed an increasing trend from NAA to AANFS and then to AAFS. Children and adults with early onset asthma had greater % of AAFS than adults with late onset asthma (46% and 40% vs. 32%; P < 0.00001). In children, AAFS and AANFS had lower % predicted FEV1 (86% and 91% vs. 97%) and greater % of patients with severe asthma than NAA (61% and 59% vs. 43%). In adults with early or late onset asthma, NAA had greater % of patients with severe asthma than AANFS and AAFS (61% vs. 40% and 37% or 56% vs. 44% and 49%). The G allele of rs2872507 in GSDMB had higher frequency in AAFS than AANFS and NAA (0.63 vs. 0.55 and 0.55), and associated with earlier age onset and asthma severity.
Conclusions:
Early or late onset AAFS, AANFS, and NAA have shared and distinct phenotypic characteristics in children and adults. AAFS is a complex disorder involving genetic susceptibility and environmental factors.
Keywords: Age of asthma onset, asthma subphenotypes, atopic asthma with fungal sensitization, GSDMB, non-atopic asthma, severe asthma
Introduction
Asthma is a heterogeneous respiratory complex disease. Subphenotypes of asthma can be determined by age of asthma onset [1–5]. Early onset asthma tends to be more atopic with family history of atopy or asthma; late onset asthma is more likely to be non-atopic, female, and smokers [1–5]. Asthma can also be classified by atopic status, such as atopic asthma with fungal sensitization (AAFS), atopic asthma with non-fungal sensitization (AANFS), and non-atopic asthma (NAA). AAFS (vs. AANFS) has been associated with asthma severity [6–8], respiratory arrest [9], admission to intensive care unit [10–11], emergency department visits [12–13], hospitalizations [14–15], frequent asthma exacerbations [16], and asthma-related mortality [17]. However, clinical manifestations of AAFS, AANFS, and NAA and how they are associated with asthma severity may be inconsistent in children and adults [18–20]. Importantly, the overlaps between early onset and atopic asthma or between late onset and non-atopic asthma are not completely congruent; combinatory effects of age of asthma onset and atopic status have not been investigated. In this study, we comprehensively investigated and compared phenotypic characteristics of early or late onset AAFS, AANFS, and NAA in children and adults in the well-characterized SARP cohort.
Genome-wide association studies (GWASs) have indicated that early onset asthma may have a greater genetic component and reduced environmental component than late onset asthma [21–23]. GWASs further confirmed that single nucleotide polymorphisms (SNPs) in chr17q12–21 region is associated with early onset but not late onset asthma [21–23]. Fungal sensitization is mediated by Th2-driven adaptive immune response and group 2 innate lymphoid cells (ILC2)-driven innate immune response [24–26]. Th1 and Th17 responses are also involved in severe asthma with fungal sensitization (SAFS) [24–25,27]. Up to now, no GWAS has been performed to investigate SNPs associated with AAFS, AANFS, and NAA, mainly due to limited sample size [28–29]. In this study, we performed genetic association analyses of fungal sensitivity, asthma severity, and age of asthma onset with six most consistently replicated SNPs/genes (GSDMB, HLA-DRA, IL13, IL1RL1, IL33, and TSLP) identified through GWASs of asthma [28,30]. We hypothesize that genetic predisposition and environmental exposure are essential for AAFS development and progression. Shared and distinct phenotypic characteristics of early or late onset AAFS, AANFS, and NAA in children and adults will be revealed using the comprehensively phenotyped SARP cohort.
Methods
Study participants
SARP is a currently active NHLBI-sponsored program involving patients with mild to severe asthma and a subset of controls. For the SARP longitudinal cohort and cross-sectional cohort, non-smokers with asthma based on the ATS-ERS criteria [31] were recruited [32–36]. Participants in SARP were comprehensively phenotyped [2, 32–41]. SARP was approved by the appropriate institutional review board including informed consent
Early or late onset asthma (age of asthma onset < 12 years or ≥ 12 years) with atopic status in children (6 < age < 18 years) and adults (age ≥ 18 years) were included in this study. asthma were not included due to very small sample size (n = 5). Skin prick test (SPT) was available in the cross-sectional cohort (n = 1,042 asthmatics and 255 adult controls), including 12 allergens (Aspergillus fumigatus, Cladosporium herbarum, Alternaria alternate, Dermatophagoides farinae, Dermatophagoides pteronyssinus, eastern tree mix, southern grass mix, ragweed short AgE, weed mix, cockroach mix, cat hair, and dog epithelia/mixed breeds). Serum specific IgE (sIgE) was measured in the longitudinal cohort (n = 702 asthmatics), including 15 allergens (Aspergillus fumigatus, Cladosporium herbarum, Alternaria alternate, Dermatophagoides farinae, Dermatophagoides pteronyssinus, tree mix Tx4, tree mix Tx6, grass mix Gx2, ragweed, weed mix Wx1, cockroach, cat dander, dog dander, mouse urine proteins, and rat urine proteins). A positive response was defined as a wheal ≥ 3 mm diameter after subtraction of the negative diluent control for SPT or as sIgE levels ≥ 0.35 kU/L. AAFS was defined as positive response to at least one fungal allergen (Aspergillus fumigatus, Cladosporium herbarum, and Alternaria alternate) who may be also sensitive to non-fungal allergens; AANFS was defined as positive response to at least one non-fungal allergen but not to any fungal allergen; NAA was defined as negative response to all allergens being tested. SARP cross-sectional and longitudinal cohorts are two independent studies recruited at different time periods; 71 asthmatics (68 adults and 3 children) were included in both cohorts and removed from the cross-sectional cohort in the final merged dataset (1,673 asthmatics and 255 controls).
Statistical analyses
Clinical characteristics were presented as means with standard deviations for normally distributed continuous variables, medians with the first and third quartiles for non-normally distributed continuous variables, and counts with percentages for categorical variables. Phenotypic comparisons were performed for continuous and categorical variables using Kruskal-Wallis test and chi-square test, respectively. Analyses of clinical variables were performed using SAS software version 9.4 (SAS Institute Inc., Cary, NC), and nominal p-value of 0.05 was considered as significant.
Genetic association analyses of six asthma candidate SNPs (rs2872507, rs2395185, rs20541, rs13431828, rs3939286, and rs1837253) extracted from TOPMed whole-genome sequencing (dbGaP accession: phs001446) were performed using PLINK [42]. Logistic or linear regression, assuming a genetic additive model, was used for association analyses of fungal sensitivity (AAFS [n = 233] vs. AANFS [n = 385], AAFS vs. NAA [n = 169], and AANFS vs. NAA), asthma severity (345 severe vs. 442 non-severe asthma), and age of asthma onset (n = 787) in SARP non-Hispanic White adults, adjusted for age, sex, and the first five principal components as described [34]. Nominal p-value of 0.05 was considered as significant.
Results
Participant demographics and asthma susceptibility
Adults with early onset (n = 683) or late onset (n = 670) asthma, children with early onset asthma (n = 320), and healthy controls of adults (n = 255) stratified by atopic status as AAFS (atopic fungal (+) asthma), AANFS (atopic fungal (−) asthma), and NAA were presented as Venn diagram (Fig. S1) and in Table 1. Adults with early or late onset asthma and children with early onset asthma all showed significant higher % of atopic fungal (+) and atopic fungal (−) but lower % of non-atopic than healthy controls (P < 0.00001), indicating that atopy is a key risk factor for asthma susceptibility. Adults with early onset asthma showed significant higher % of AAFS (40% vs. 24%) but lower % of NAA (9% vs. 24%) than adults with late onset asthma (P < 0.00001), indicating that early and late onset asthma might represent two asthma subphenotypes. Adults with early onset asthma showed significant (P = 0.017) but relatively similar % of AAFS (40% vs. 46%), AANFS (51% vs. 41%), and NAA (9% vs. 13%) compared with children, indicating that a large proportion of children with early onset AAFS, AANFS, and NAA might transit into adults with early onset AAFS, AANFS, and NAA, respectively.
Table 1.
Age of asthma onset and atopic status in children and adults.
| Non-Atopic | Atopic Fungal (—) | Atopic Fungal (+) | P value | |
|---|---|---|---|---|
| Healthy Controls (Adults; n=255) | 152 (59.6%) | 75 (29.4) | 28 (11.0%) | |
| Late Onset Asthma (Adults; n=670) | 163 (24.3%) | 347 (51.8%) | 160 (23.9%) | <0.00001† |
| Early Onset Asthma (Adults; n=683) | 62 (9.1%) | 345 (50.5%) | 276 (40.4%) | <0.00001‡ |
| Early Onset Asthma (Children; n=320) | 40 (12.5%) | 132 (41.3%) | 148 (46.3%) | 0.017§ |
Note: number and % of participants were shown.
2×3 chi-square test of Healthy Controls (Adults; n=255) vs. Late Onset Asthma (Adults; n=670).
2×3 chi-square test of Late Onset Asthma (Adults; n=670) vs. Early Onset Asthma (Adults; n=683).
2×3 chi-square test of Early Onset Asthma (Adults; n=683) vs. Early Onset Asthma (Children; n=320).
Demographics of SARP participants stratified by age of asthma onset and atopic status in children and adults were presented in Table S1. Age of asthma onset was significantly earlier in AAFS than AANFS and NAA in adults with early onset asthma (4.1 vs. 4.7 and 4.8 years) and significantly later in NAA than AANFS and AAFS (33 vs. 27 and 25 years) in adults with late onset asthma. Age of asthma onset was also earlier in AAFS than AANFS and NAA in children (2.2 vs. 2.6 and 2.6 years), though not statistically significant. More females were presented in adults with asthma, especially with late onset asthma, than children with early onset asthma.
Pulmonary function and asthma severity
Pulmonary function and asthma severity were presented as Figure 1 and in Table 2. Airway hyper-responsiveness (AHR; measured by PC20 in a subset of SARP participants) showed an increasing trend from NAA to AANFS then to AAFS in children (4.5 to 1.3 to 0.6), adults with early onset asthma (2.6 to 1.1 to 0.9), and adults with late onset asthma (2.5 to 1.6 to 1.1), indicating that patients with AAFS tend to have greater AHR. In children, AAFS and AANFS had lower baseline pre-bronchodilator (pre-BD) % predicted FEV1 (86% and 91% vs. 97%) and greater % of patients with severe asthma than NAA (61% and 59% vs. 43%). In adults with early or late onset asthma, NAA had greater % of patients with severe asthma than AANFS and AAFS (61% vs. 40% and 37% or 56% vs. 44% and 49%) and greater % of patients with exacerbations in the last 12 months (45% vs. 24% and 22% or 35% vs. 26% and 23%), however, baseline % predicted FEV1 was similar between NAA, AANFS, and AAFS. In children and adults with early onset asthma, NAA had higher FEV1/FVC and lower FEV1 reversibility than AANFS and AAFS. In summary, AAFS, AANFS, and NAA showed distinct patterns for pulmonary function and asthma severity in children and adults, i.e., increased asthma severity was associated with atopy in children but associated with non-atopy in adults.
Figure 1.
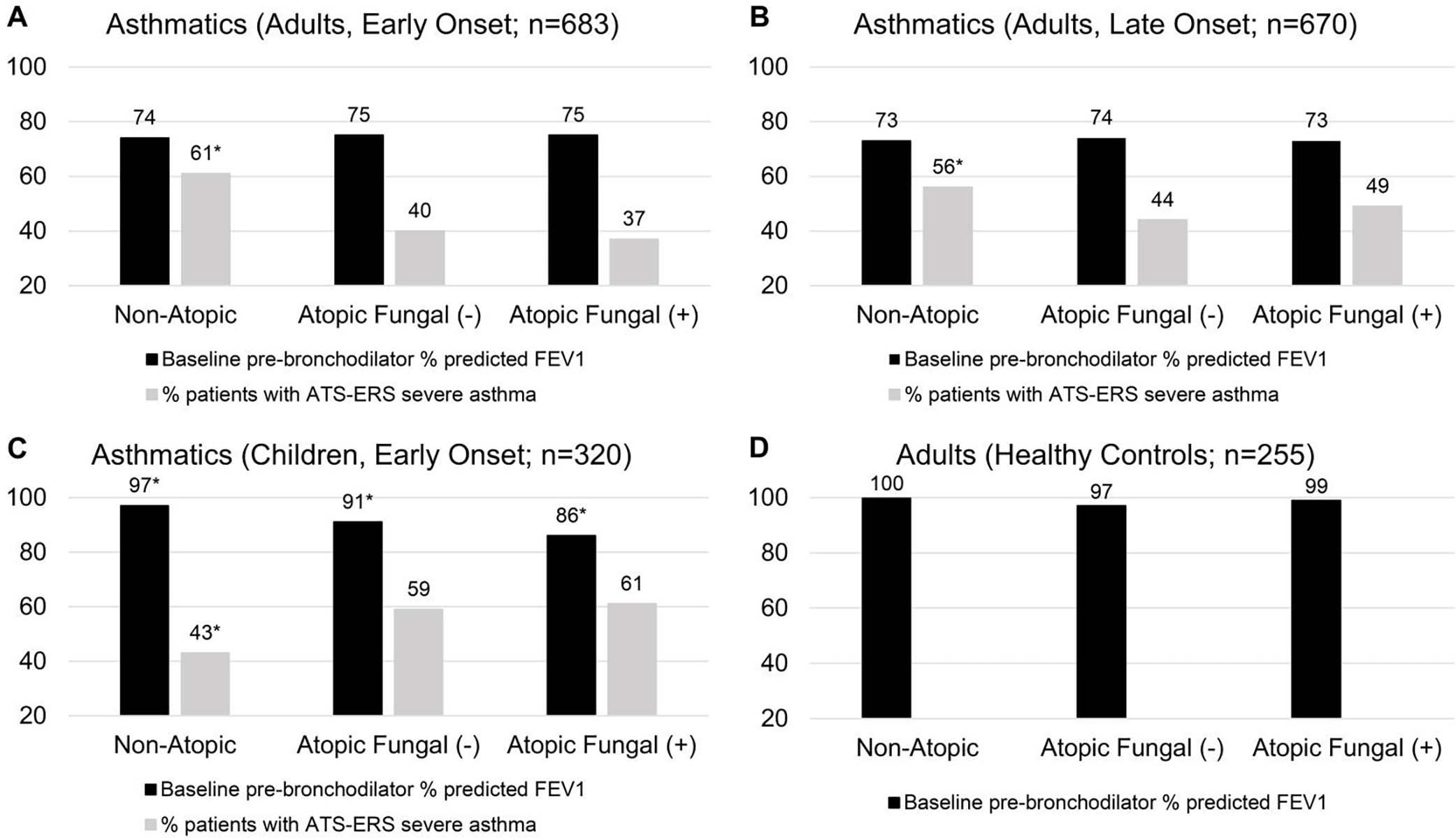
Pulmonary function and asthma severity of participants stratified by age of asthma onset and atopic status in children and adults. *Baseline % predicted FEV1 or % patients with severe asthma for the specific group was significantly different from other groups in the same panel.
Table 2.
Pulmonary function and asthma severity stratified by age of asthma onset and atopic status in children and adults.
| Children, Early Onset (n=320) | NAA | AANFS | AAFS | P value | |||||
|---|---|---|---|---|---|---|---|---|---|
| N | Mean ± SD | N | Mean ± SD | N | Mean ± SD | AAFS vs. AANFS | AAFS vs. NAA | AANFS vs. NAA | |
| Baseline FEV1 % predicted | 40 | 97 ± 12 | 132 | 91 ± 17 | 148 | 86 ± 19 | 0.014 | 0.0002 | 0.047 |
| Baseline FVC % predicted | 40 | 103 ± 12 | 132 | 102 ± 14 | 148 | 100 ± 17 | 0.15 | 0.18 | 0.87 |
| Baseline FEV1/FVC | 40 | 0.82 ± 0.07 | 132 | 0.78 ± 0.10 | 148 | 0.74 ± 0.10 | 0.0013 | <0.0001 | 0.02 |
| Maximal FEV1 % predicted | 39 | 107 ± 12 | 132 | 105 ± 15 | 148 | 102 ± 17 | 0.14 | 0.038 | 0.28 |
| Maximal FVC % predicted | 39 | 108 ± 13 | 130 | 110 ± 15 | 148 | 109 ± 17 | 0.72 | 061 | 0.37 |
| Maximal Reversibility | 39 | 13 ± 10 | 132 | 18 ± 19 | 148 | 21 ± 17 | 0.01 | 0.0035 | 0.33 |
| PC20 methacholine, mg/mL | 19 | 4.5 (1.1, 9.5) | 70 | 1.3 (0.6, 4.0) | 61 | 0.6 (0.3, 2.0) | 0.014 | 0.001 | 0.028 |
| ATS-ERS severe asthma, n (%) | 40 | 17 (43) | 132 | 78 (59) | 148 | 91 (61) | 0.71 | 0.047 | 0.072 |
| Exacerbations in the last 12 months, n (%) | 40 | 22 (55) | 130 | 70 (54) | 148 | 84 (57) | 0.63 | 0.86 | 1 |
| ACQ-6 | 13 | 1.31 ± 1.11 | 71 | 1.23 ± 1.16 | 92 | 1.32 ± 1.37 | 0.95 | 0.75 | 0.68 |
| Adults, Early Onset (n=683) | NAA | AANFS | AAFS | P value | |||||
| N | Mean ± SD | N | Mean ± SD | N | Mean ± SD | AAFS vs. AANFS | AAFS vs. NAA | AANFS vs. NAA | |
| Baseline FEV1 % predicted | 62 | 74 ± 24 | 345 | 75 ± 21 | 276 | 75 ± 21 | 0.65 | 0.86 | 0.69 |
| Baseline FVC % predicted | 60 | 82 ± 19 | 344 | 88 ± 19 | 274 | 89 ± 18 | 0.69 | 0.017 | 0.026 |
| Baseline FEV1/FVC | 60 | 0.72 ± 0.14 | 344 | 0.70 ± 0.11 1 | 275 | 0.69 ± 0.12 | 0.33 | 0.017 | 0.059 |
| Maximal FEV1 % predicted | 61 | 84 ± 22 | 343 | 88 ± 19 | 272 | 87 ± 19 | 0.67 | 0.45 | 0.31 |
| Maximal FVC % predicted | 59 | 90 ± 16 | 342 | 97 ± 17 | 272 | 97 ± 16 | 0.64 | 0.0013 | 0.0038 |
| Maximal Reversibility | 61 | 16 ± 19 | 343 | 19 ± 20 | 272 | 18 ± 18 | 0.69 | 0.02 | 0.012 |
| PC20 methacholine, mg/mL | 29 | 2.6 (0.6, 4.9) | 196 | 1.1 (0.4, 3.4) | 167 | 0.9 (0.3, 2.7) | 0.16 | 0.02 | 0.09 |
| ATS-ERS severe asthma, n (%) | 62 | 38 (61) | 345 | 137 (40) | 276 | 103 (37) | 0.56 | 0.0009 | 0.002 |
| Exacerbations in the last 12 months, n (%) | 62 | 28 (45) | 343 | 81 (24) | 276 | 62 (22) | 0.77 | 0.0004 | 0.0009 |
| ACQ-6 | 27 | 1.74 ± 1.29 | 122 | 1.55 ± 1.37 | 97 | 1.20 ± 1.14 | 0.06 | 0.038 | 0.34 |
| Adults, Late Onset (n=670) | NAA | AANFS | AAFS | P value | |||||
| N | Mean ± SD | N | Mean ± SD | N | Mean ± SD | AAFS vs. AANFS | AAFS vs. NAA | AANFS vs. NAA | |
| Baseline FEV1 % predicted | 163 | 73 ± 23 | 347 | 74 ± 20 | 160 | 73 ± 21 | 0.61 | 0.73 | 0.43 |
| Baseline FVC % predicted | 163 | 82 ± 19 | 347 | 84 ± 17 | 159 | 85 ± 18 | 0.67 | 0.093 | 0.13 |
| Baseline FEV1/FVC | 163 | 0.70 ± 0.12 | 347 | 0.70 ± 0.12 | 159 | 0.69 ± 0.12 | 0.16 | 0.26 | 0.95 |
| Maximal FEV1 % predicted | 163 | 83 ± 21 | 346 | 86 ± 19 | 159 | 86 ± 20 | 0.89 | 0.13 | 0.085 |
| Maximal FVC % predicted | 163 | 89 ± 17 | 346 | 93 ± 16 | 159 | 95 ± 15 | 0.09 | 0.0006 | 0.02 |
| Maximal Reversibility | 163 | 17 ± 18 | 346 | 17 ± 17 | 159 | 18 ± 16 | 0.33 | 0.29 | 0.85 |
| PC20 methacholine, mg/mL | 71 | 2.5 (0.8, 5.8) | 181 | 1.6 (0.5, 4.8) | 85 | 1.1 (0.4, 3.7) | 0.31 | 0.016 | 0.08 |
| ATS-ERS severe asthma, n (%) | 163 | 92 (56) | 347 | 151(44) | 160 | 78 (49) | 0.29 | 0.18 | 0.0077 |
| Exacerbations in the last 12 months, n (%) | 163 | 57 (35) | 346 | 91 (26) | 160 | 37 (23) | 0.51 | 0.02 | 0.047 |
| ACQ-6 | 84 | 1.43 ± 1.43 | 135 | 1.16 ± 1.20 | 55 | 1.27 ± 1.10 | 0.34 | 0.87 | 0.27 |
NAA: non-atopic asthma; AANFS: atopic asthma with non-fungal sensitization; AAFS: atopic asthma with fungal sensitization.
Corticosteroids use, health care utilization, and comorbidities
In adults with early or late onset asthma, NAA had significant higher % use of high-dose inhaled corticosteroids (ICS), oral CS (OCS), and injectable CS than AANFS and AAFS (Table S2); in children, NAA had lower % use of OCS than AANFS and AAFS, which further indicated that increased asthma severity was associated with atopic and non-atopic status in children and adults, respectively.
In adults with early or late onset asthma, NAA showed significant higher % health care utilization (HCU) than AANFS and AAFS, such as ≥ 1 urgent visit/year, emergency department (ED) visit for breathing problem, ≥ 3 OCS burst/year, and hospitalizations (Table S3). In children, HCU was similar between NAA, AANFS, and AAFS.
In adults with early or late onset asthma, NAA showed significant higher % comorbidities than AANFS and AAFS, including pneumonia, bronchitis, sinusitis, nasal polyps, and gastroesophageal reflux (Table S4). In children, NAA showed higher % of pneumonia and gastroesophageal reflux than AANFS and AAFS.
T2 and non-T2 biomarkers
Total serum IgE levels showed an increasing trend from NAA to AANFS and then to AAFS in early and late onset asthma in children and adults (Table S5). Blood eosinophils and FeNO levels showed an increasing trend from NAA to AANFS and to AAFS in children and adults with early onset asthma. In children, sputum % eosinophils was significantly higher in AAFS than AANFS and NAA (3.0% vs. 0.6% and 0.6%). In adults with early onset asthma, blood neutrophils showed a decreasing trend from NAA to AANFS and then to AAFS (4,542 vs. 4,000 vs. 3,600 cells/μL) and NAA had higher IL-6 levels than AANFS and AAFS (2.6 vs. 1.6 and 1.4 pg/mL). In adults with late onset asthma, NAA had lower FeNO levels than AANFS and AAFS (21 vs. 26 and 24 ppb).
Combinations of T2 and non-T2 biomarkers may reveal asthma subphenotypes and underlying biological mechanisms [37,41]. In adults with early onset asthma, a combination of FeNO (cutoff = 25 ppb) and IL-6 (cutoff = 3.1 pg/mL) or blood eosinophils (cutoff = 300 cells/μL) and blood neutrophils (cutoff = 4,000 cells/μL) distinguished AAFS, AANFS, and NAA (Table S6 and Fig. S2). In children with early onset asthma, a combination of blood eosinophils and blood neutrophils distinguished AAFS, AANFS, and NAA.
Genetic association analyses of six candidate SNPs/genes
For six candidate SNPs tested in this study, rs2395185 in HLA-DRA and rs2872507 in GSDMB were significantly associated with fungal sensitivity (Table 3). The T allele of rs2395185 in HLA-DRA had higher frequency in AAFS than AANFS and NAA (0.39 vs. 0.34 and 0.31), indicating that T allele was the minor risk allele for AAFS. The G allele of rs2872507 in GSDMB had higher frequency in AAFS than AANFS and NAA (0.63 vs. 0.55 and 0.55), indicating that G allele was the major risk allele for AAFS.
Table 3.
Genetic association of six candidate SNPs with fungal sensitivity.
| SNP | Gene | Chr | Location | Allele† | MAF‡ | P value (Odds Ratio) | ||
|---|---|---|---|---|---|---|---|---|
| AAFS vs. AANFS | AAFS vs. NAA | AANFS vs. NAA | ||||||
| rs13431828 | IL1RL1 | 2 | 5' UTR | C/T | 0.13/0.10/0.12/0.13 | 0.42 (1.17) | 0.27 (0.78) | 0.15 (0.75) |
| rs1837253 | TSLP | 5 | Flanking 5' | C/T | 0.22/0.22/0.21/0.26 | 0.64 (0.94) | 0.60 (0.90) | 0.97 (0.99) |
| rs20541 | IL13 | 5 | Missense | G/A | 0.18/0.19/0.21/0.18 | 0.44 (1.12) | 0.25 (1.26) | 0.67 (1.08) |
| rs2395185 | HLA-DRA | 6 | Flanking 3' | G/T | 0.31/0.34/0.39/0.33 | 0.062 (1.26) | 0.017 (1.47) | 0.22 (1.20) |
| rs3939286 | IL33 | 9 | Flanking 5' | C/T | 0.29/0.28/0.31/0.26 | 0.35 (1.13) | 0.43 (1.14) | 0.95 (1.01) |
| rs2872507 | GSDMB | 17 | Flanking 3' | G/A | 0.45/0.45/0.37/0.45 | 0.0099 (0.73) | 0.010 (0.66) | 0.80 (0.97) |
NAA: non-atopic asthma; AANFS: atopic asthma with non-fungal sensitization; AAFS: atopic asthma with fungal sensitization.
Allele: Major/Minor allele
MAF: minor allele frequency in 169 NAA, 385 AANFS, 233 AAFS, and ~4,300 general North-Western Europeans from gnomAD V2.1.1 (https://gnomad.broadinstitute.org/).
In a logistic regression model (Table S7), the G allele of rs2872507 in GSDMB (OR = 1.25; P = 0.036) and non-atopy (OR = 1.23; P = 0.038) were associated with asthma severity. In a linear regression model, the G allele of rs2872507 in GSDMB (β = −1.71; P = 0.027) and fungal sensitization (β = −5.68; P < 0.0001) were associated with earlier age of asthma onset. In summary, a combination of GG genotype of rs2872507 and fungal sensitization were associated with earliest age of asthma onset; a combination of GG genotype of rs2872507 and non-atopy were associated with highest % of patients with severe asthma (64%) (Fig. S3).
Discussion
Our findings on demographics, pulmonary function, corticosteroids use, health care utilization, comorbidities, T2/non-T2 biomarkers, genetics, asthma susceptibility and asthma severity in SARP indicated that children/adults with early onset or adults with late onset AAFS, AANFS, and NAA might represent six asthma subphenotypes (Fig. S4). Within each asthma subphenotypes, asthmatics with non-severe asthma may further progress into severe asthma. For example, severe asthma with fungal sensitization (SAFS), a previously reported asthma subphenotype, is an extreme subset of AAFS [43].
Children and adults with early onset asthma shared some key clinical characteristics, including similar distribution of atopic status (NAA, AANFS, and AAFS) (Table 1 and Fig. S1) and increasing trend of T2 biomarkers (total serum IgE levels, blood eosinophils, and FeNO levels) from NAA to AANFS and then to AAFS (Table S5). However, children and adults with early onset asthma also showed some distinct difference, such as % of females or males (Table S1) and % of severe asthma in NAA, AANFS, and AAFS (Table 2 and Fig. 1). Theoretically, adults with early onset asthma may be the grown-up (persistence) or relapse from children with early onset asthma; however, a large proportion of children with asthma may lose asthma symptoms during development (remission) [4,44–45]. In this study, males were predominant (59%) in children and female were predominant (61%) in adults with early onset asthma (Table S8), which agrees with previous findings. This gender difference in asthma may be due to remission of asthma in more boys and new onset of asthma in more girls during school age or adolescence [5,46]. In this study, increased asthma severity was associated with AAFS and AANFS in children but associated with NAA in adults with early onset asthma (Table 2. Since children and adults with early onset asthma were cross-sectional in SARP, the mechanism of this asthma severity transition can only be answered by future large longitudinal epidemiology studies. Some probable reasons are: 1) more children with non-severe NAA progress into adults with severe early onset NAA; 2) more children with severe AAFS or AANFS remit or transit into adults with early onset atopic asthma but symptoms become milder; 3) definition of asthma and/or severe asthma are different between children and adults.
Adults with early or late onset asthma also shared some clinical characteristics. For example, NAA had higher % of corticosteroids use (Table S2), HCU (Table S3), comorbidities (Table S4), and severe asthma (Table 2) than AANFS and AAFS, which may be due to consistent definition of asthma and severe asthma in adults and shared mechanisms of atopy. In addition to different age of asthma onset, adults with early or late onset asthma showed distinct difference in distribution of atopic status (NAA, AANFS, and AAFS) and genetics. Similar to previous findings, adults with late onset asthma had higher % of NAA (24% vs. 9%) and lower % of AAFS (24% vs. 40%) than adults with early onset asthma (Table 1 and Fig. S1). Adults with early onset asthma had greater burden of genetic factors than adults with late onset asthma (Table 3, Table S7, and Fig. S3). For example, The G allele of rs2872507 in GSDMB was risk allele for AAFS, early onset asthma, and asthma severity in this study, which has been also associated with asthma susceptibility in previous genetic studies [28,30].
One of the great advantages of this study is the utilization of extremely well-characterized SARP cohorts. By using SARP cohorts, we have the opportunity for the first time to categorize patients with asthma into nine groups based on age of asthma onset and atopic status. Only through this approach, clinical characteristics of six asthma subphenotypes (Fig. S4) can be comprehensively investigated and compared in children and adults. If either age of asthma onset (early vs. late onset) or atopic status (AAFS vs. AANFS vs. NAA) were used individually for asthma categorization as some previous studies did [2,47], then only one facet of asthma subphenotypes with limited or even biased information could be generated. For example, clinical characteristics stratified only by age of asthma onset (with different atopic status mixed) (Table S8) or only by atopic status (with different age of asthma onset in children and adults mixed) in healthy controls (Table S9) and in asthmatics (Table S10) were presented for convenience. In addition to age of asthma onset and atopic status, further categorization by gender (male or female) was presented in Table S11. In adults with late onset NAA, males showed later age of asthma onset (39 vs. 31 years), lower BMI (29 vs. 33), higher T2 biomarkers (total serum IgE levels (34 vs. 21 IU/mL), FeNO (30 vs. 18 ppb), and blood eosinophils (300 vs. 186 cells/μL)), higher % of high-dose ICS (76% vs. 52%), lower baseline pre-BD % predicted FEV1 (64% vs. 76%) and greater % of patients with severe asthma (70% vs. 52%) than females. Lower baseline pre-BD % predicted FEV1 in males was also shown in adults with early or late onset AANFS and AAFS.
There are some shortcomings or unanswered questions in this study. SPT and sIgE were used to define atopy in the cross-sectional cohort and the longitudinal cohort, respectively. Similar to previous reports, consistency of atopy defined by SPT and sIgE (measured in 68 adults in SARP) was not perfect with % of agreement ranging from 63% (dog dander) to 88% (grass mix) (Table S12). To enlarge sample size for the investigations of asthma subphenotypes, the cross-sectional and longitudinal cohorts were merged. It is very difficult or almost impossible to dissect the effect of single allergen in human studies because most of the studied participants are allergic to more than one allergen. Association analysis of single allergen and asthma severity was performed by comparing participants with positive or negative (including positive response to other allergens and NAA) response to a specific allergen (Table S13). In adults with early onset asthma, positive responses to Aspergillus fumigatus and dog dander were associated with higher % of severe asthma; positive responses to ragweed, weed mix, grass mix, and house dust mite (Dermatophagoides farina and Dermatophagoides pteronyssinus) were associated with lower % of severe asthma. In adults with late onset asthma, positive responses to ragweed, weed mix, grass mix, tree mix, and house dust mite were associated with lower % of severe asthma. In this study, AAFS was defined as positive response to at least one fungal allergen, same as previous studies (most were also allergic to non-fungal allergens; Fig. S1). AAFS was further divided into participants allergic only to fungal allergens (2%) and allergic to both fungal and non-fungal allergens (Table S14). In adults with late onset asthma, patients allergic only to fungal allergens showed significant higher % severe asthma than patients allergic to both fungal and non-fungal allergens, AANFS, and NAA (85% vs. 46%, 44%, and 56%), indicating that positive fungal sensitivity is a risk factor for asthma severity. In children and adults with early onset asthma, patients allergic only to fungal allergens also showed higher % severe asthma than patients allergic to both fungal and non-fungal allergens, though not statistically significant due to small sample size.
In summary, total serum IgE levels and AHR showed a consistent increasing trend from NAA to AANFS then to AAFS in early or late onset asthma in children and adults (Table 2 and Table S5). A genetic variant in GSDMB and fungal sensitivity were associated with earlier age of asthma onset and the same genetic variant in GSDMB and non-atopy were associated with asthma severity in adults (Fig. S3 and Table S7). In a previous GWAS, we have shown that genes associated with airway structure/remodeling and Th1 pathway were associated with lower lung function and asthma severity [48]. In a candidate gene study, we have shown that SNPs in GSDMB were associated with asthma severity and asthma exacerbations probably through antiviral pathways [34]. On the basis of these findings, we suggest a two-step gene-environment interaction fungal asthma progression model (Fig. S5). In the first step, genetic variants (such as GSDMB) associated with atopy, Th2 pathway, and lung function, interacting with environmental factors (fungi and other allergens), induce Th2-dominant atopic response, which leads to childhood onset fungal asthma. In the second step, genetic variants (such as GSDMB) associated with Th2 pathway, Th1 pathway, and lung function, interacting with environmental factors (fungi and virus) lead to severe asthma with fungal sensitization in children and adults. In the future, personalized treatment approaches may be investigated and pursued in these asthma subphenotypes.
Supplementary Material
Acknowledgements
We acknowledge all investigators, staff, and participants in the SARP studies. The following companies provided financial support for study activities at the Coordinating and Clinical Centers beyond the third year of patient follow-up: AstraZeneca, Boehringer-Ingelheim, Genentech, GlaxoSmithKline, Sanofi–Genzyme–Regeneron, and TEVA.
Funding
This study was supported by NIH grant AI149754. SARP cross-sectional cohort (stage 1 and 2; SARP1–2) was supported by NIH grants HL69116, HL69130, HL69149, HL69155, HL69167, HL69170, HL69174, HL69349, UL1RR024992, M01RR018390, M01RR07122, M01RR03186, HL087665, and HL091762. SARP longitudinal cohort (stage 3; SARP3) was funded by the NHLBI U10 HL109172, HL109168, HL109152, HL109257, HL109146, HL109250, HL109164, and HL109086 and the Clinical and Translational Science Awards (CTSA) Program UL1 TR001102, UL1 TR000427, UL1 TR001420, and UL1 TR002378. SARP longitudinal cohort (stage 4; SARP4) was funded by NIH HL146002. Genetic studies for SARP cross-sectional cohort were funded by NIH HL87665 and Go Grant RC2HL101487. SARP whole-genome sequencing was supported by National Heart, Lung, and Blood Institute (NHLBI) Trans-Omics for Precision Medicine (TOPMed) X01 grant. This work was also supported by the NIH grants HL142769. The following companies provided financial support for study activities at the Coordinating and Clinical Centers beyond the third year of patient follow-up: AstraZeneca, Boehringer-Ingelheim, Genentech, GlaxoSmithKline, Sanofi–Genzyme–Regeneron, and TEVA. These companies had no role in study design or data analysis, and the only restriction on the funds was that they be used to support the SARP initiative.
Footnotes
Declaration of interest: Dr. Castro receives University Grant Funding from NIH, American Lung Association, PCORI; he receives Pharmaceutical Grant Funding from AstraZeneca, GSK, Novartis, Pulmatrix, Sanofi-Aventis, Shionogi; he is a consultant for Genentech, Teva, Sanofi-Aventis, Merck, Novartis; he is a speaker for Amgen, AstraZeneca, Genentech, GSK, Regeneron, Sanofi, Teva; he receives Royalties from Elsevier. Dr. Denlinger receives University Grant Funding from NHLBI; he also receives PrecISE and GRAIL grants from NHLBI and Lung Health Cohort grant from ALA-ACRC which are not related to SARP; he receives drugs for the NHLBI PrecISE trial from GlaxoSmithKline, Laurel, Sun Pharma, Vifor, Vitaeris/CSL Behring, Vitaflo and equipment contracts with the NHLBI PrecISE trial from Vyaire, Caire Diagnostics, MIR, Propeller Health, ZEPHYRx. Dr. Gaston is founder and equity owner in Respiratory Research Incorporated as well as Airbase Breathing Company, LLC; his lab is funded by the NIH, the Eli Lilly Foundation, the Harrington Discovery Institute, and the Riley Children’s Foundation. Dr. Jarjour receives University Grant Funding from NHLBI; he is a consultant for AstraZeneca, GSK. Dr. Levy reports grants from NHLBI, personal consulting fees from AstraZeneca, Bayer, Gossamer Bio, NControl, Novartis, Pieris Pharmaceuticals, Sanofi, Teva, participates on NIAID/DAIT COVID-19 DSMB board, and has stock from Entrinsic Biosciences, Nocion Therapeutics. Dr. Mauger reports grants from NHLBI and drugs for NIH-funded clinical trials from Genentech, GSK, OM Pharma, Sanofi-Regeneron. Dr. Wenzel receives Pharmaceutical Grant Funding from AstraZeneca, Knopp, Regeneron; she is a consultant for AstraZeneca, Knopp, Novartis, Sanofi, GSK; she is on scientific advisory board of Aer Therapeutics. All other authors have nothing to disclose. Dr. Bleecker reports grants from NHLBI and Pharmaceutical Grant Funding from NHLBI; he is a consultant for AstraZeneca, GSK. Dr. Levy reports grants from NHLBI, personal consulting fees from AstraZeneca, Bayer, Gossamer Bio, NControl, Novartis, Pieris Pharmaceuticals, Sanofi, Teva, participates on NIAID/DAIT COVID-19 DSMB board, and has stock from Entrinsic Biosciences, Nocion Therapeutics. Dr. Mauger reports grants from NHLBI and drugs for NIH-funded clinical trials from Genentech, GSK, OM Pharma, Sanofi-Regeneron. Dr. Wenzel receives Pharmaceutical Grant Accepted Manuscript Funding from AstraZeneca, Knopp, Regeneron; she is a consultant for AstraZeneca, Knopp, Novartis, Sanofi, GSK; she is on scientific advisory board of Aer Therapeutics. All other authors have nothing to disclose. Dr. Bleecker reports grants from NHLBI and Pharmaceutical Grant Funding from AstraZeneca, Novartis, Regeneron, Sanofi Genzyme, and clinical trials administrated through University of Arizona; he is a consultant for AstraZeneca, Glaxo Smith Kline, Knopp Pharmaceuticals, Novartis, Regeneron, and Sanofi Genzyme; he receives payment for lectures from AstraZeneca and support for travel from AstraZeneca, Glaxo Smith Kline, Novartis, Regeneron, and Sanofi Genzyme. Dr. Meyers reports grants from NHLBI and Pharmaceutical Grant from GSK; she receives consulting fees from AstraZeneca. The rest of the authors declare that they have no relevant conflict of interest.
Supplemental online material
Additional supplemental online material may be found in the online version of this article.
References
- 1.Miranda C, Busacker A, Balzar S, Trudeau J, Wenzel SE. Distinguishing severe asthma phenotypes: role of age at onset and eosinophilic inflammation. J Allergy Clin Immunol 2004;113:101–108. [DOI] [PubMed] [Google Scholar]
- 2.Moore WC, Bleecker ER, Curran-Everett D, Erzurum SC, Ameredes BT, Bacharier L, et al. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. J Allergy Clin Immunol 2007;119:405–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan DJ, Walters EH, Perret JL, Lodge CJ, Lowe AJ, Matheson MC, et al. Age-of-asthma onset as a determinant of different asthma phenotypes in adults: a systematic review and meta-analysis of the literature. Expert Rev Respir Med 2015;9:109–123. [DOI] [PubMed] [Google Scholar]
- 4.Fuchs O, Bahmer T, Rabe KF, von Mutius E. Asthma transition from childhood into adulthood. Lancet Respir Med 2017;5:224–234. [DOI] [PubMed] [Google Scholar]
- 5.Azim A, Freeman A, Lavenu A, Mistry H, Haitchi HM, Newell C, et al. New Perspectives on Difficult Asthma; Sex and Age of Asthma-Onset Based Phenotypes. J Allergy Clin Immunol Pract 2020;8:3396–3406. [DOI] [PubMed] [Google Scholar]
- 6.Zureik M, Neukirch C, Leynaert B, Liard R, Bousquet J, Neukirch F, et al. Sensitisation to airborne moulds and severity of asthma: cross sectional study from European Community respiratory health survey. BMJ 2002;325:411–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neukirch C, Henry C, Leynaert B, Liard R, Bousquet J, Neukirch F. Is sensitization to Alternaria alternata a risk factor for severe asthma? A population-based study. J Allergy Clin Immunol 1999;103:709–711. [DOI] [PubMed] [Google Scholar]
- 8.Parthasarathi A, Padukudru S, Krishna MT, Mahesh PA. Clinical characterization of asthma with fungal sensitization in a South Indian paediatric cohort. Clin Exp Allergy 2022;52:456–460. [DOI] [PubMed] [Google Scholar]
- 9.O’Hollaren MT, Yunginger JW, Offord KP, Somers MJ, O’Connell EJ, Ballard DJ, et al. Exposure to an aeroallergen as a possible precipitating factor in respiratory arrest in young patients with asthma. N Engl J Med 1991;324:359–363. [DOI] [PubMed] [Google Scholar]
- 10.Black PN, Udy AA, Brodie SM. Sensitivity to fungal allergens is a risk factor for life-threatening asthma. Allergy 2000;55:501–504. [DOI] [PubMed] [Google Scholar]
- 11.Medrek SK, Kao CC, Yang DH, Hanania NA, Parulekar AD. Fungal Sensitization Is Associated with Increased Risk of Life-Threatening Asthma. J Allergy Clin Immunol Pract 2017;5:1025–1031. [DOI] [PubMed] [Google Scholar]
- 12.Ross MA, Curtis L, Scheff PA, Hryhorczuk DO, Ramakrishnan V, Wadden RA, et al. Association of asthma symptoms and severity with indoor bioaerosols. Allergy 2000;55:705–711. [DOI] [PubMed] [Google Scholar]
- 13.Dales RE, Cakmak S, Burnett RT, Judek S, Coates F, Brook JR. Influence of ambient fungal spores on emergency visits for asthma to a regional children’s hospital. Am J Respir Crit Care Med 2000;162:2087–2090. [DOI] [PubMed] [Google Scholar]
- 14.Tham R, Vicendese D, Dharmage SC, Hyndman RJ, Newbigin E, Lewis E, et al. Associations between outdoor fungal spores and childhood and adolescent asthma hospitalizations. J Allergy Clin Immunol 2017;139:1140–1147. [DOI] [PubMed] [Google Scholar]
- 15.O’Driscoll BR, Hopkinson LC, Denning DW. Mold sensitization is common amongst patients with severe asthma requiring multiple hospital admissions. BMC Pulm Med 2005;5:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goh KJ, Yii ACA, Lapperre TS, Chan AK, Chew FT, Chotirmall SH, et al. Sensitization to Aspergillus species is associated with frequent exacerbations in severe asthma. J Asthma Allergy 2017;10:131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Targonski PV, Persky VW, Ramekrishnan V. Effect of environmental molds on risk of death from asthma during the pollen season. J Allergy Clin Immunol 1995;95:955–961. [DOI] [PubMed] [Google Scholar]
- 18.Lehmann S, Sprünken A, Wagner N, Tenbrock K, Ott H. Clinical relevance of IgE-mediated sensitization against the mould Alternaria alternata in children with asthma. Ther Adv Respir Dis 2017;11:30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sullivan A, Hunt EB, Ward C, Lapthorne S, Eustace JA, Fanning LJ, et al. The presence of Aspergillus fumigatus in asthmatic airways is not clearly related to clinical disease severity. Allergy 2020;75:1146–1154. [DOI] [PubMed] [Google Scholar]
- 20.Kids Bush A., Difficult Asthma and Fungus. J Fungi (Basel) 2020;6:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pividori M, Schoettler N, Nicolae DL, Ober C, Im HK. Shared and distinct genetic risk factors for childhood-onset and adult-onset asthma: genome-wide and transcriptome-wide studies. Lancet Respir Med 2019;7:509–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferreira MAR, Mathur R, Vonk JM, Szwajda A, Brumpton B, Granell R, et al. Genetic Architectures of Childhood- and Adult-Onset Asthma Are Partly Distinct. Am J Hum Genet 2019;104:665–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schoettler N, Rodríguez E, Weidinger S, Ober C. Advances in asthma and allergic disease genetics: Is bigger always better? J Allergy Clin Immunol 2019;144:1495–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kao CC, Hanania NA, Parulekar AD. The impact of fungal allergic sensitization on asthma. Curr Opin Pulm Med 2021;27:3–8. [DOI] [PubMed] [Google Scholar]
- 25.van Tilburg Bernardes E, Gutierrez MW, Arrieta MC. The Fungal Microbiome and Asthma. Front Cell Infect Microbiol 2020;10:583418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Castanhinha S, Sherburn R, Walker S, Gupta A, Bossley CJ, Buckley J, et al. Pediatric severe asthma with fungal sensitization is mediated by steroid-resistant IL-33. J Allergy Clin Immunol 2015;136:312–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Godwin MS, Reeder KM, Garth JM, Blackburn JP, Jones M, Yu Z, et al. IL-1RA regulates immunopathogenesis during fungal-associated allergic airway inflammation. JCI Insight 2019;4:e129055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buniello A, MacArthur JAL, Cerezo M, Harris LW, Hayhurst J, Malangone C, et al. The NHGRI-EBI GWAS Catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res 2019;47:D1005–D1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Overton NL, Simpson A, Bowyer P, Denning DW. Genetic susceptibility to severe asthma with fungal sensitization. Int J Immunogenet 2017;44:93–106. [DOI] [PubMed] [Google Scholar]
- 30.Li X, Ampleford EJ, Howard TD, Moore WC, Torgerson DG, Li H, et al. Genome-wide association studies of asthma indicate opposite immunopathogenesis direction from autoimmune diseases. J Allergy Clin Immunol 2012;130:861–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 2014;43:343–373. [DOI] [PubMed] [Google Scholar]
- 32.Denlinger LC, Phillips BR, Ramratnam S, Ross K, Bhakta NR, Cardet JC, et al. Inflammatory and Comorbid Features of Patients with Severe Asthma and Frequent Exacerbations. Am J Respir Crit Care Med 2017;195:302–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teague WG, Phillips BR, Fahy JV, Wenzel SE, Fitzpatrick AM, Moore WC, et al. Baseline Features of the Severe Asthma Research Program (SARP III) Cohort: Differences with Age. J Allergy Clin Immunol Pract 2018;6:545–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li X, Christenson SA, Modena B, Li H, Busse WW, Castro M, et al. Genetic analyses identify GSDMB associated with asthma severity, exacerbations, and antiviral pathways. J Allergy Clin Immunol 2021;147:894–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med 2010;181:315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jarjour NN, Erzurum SC, Bleecker ER, Calhoun WJ, Castro M, Comhair SA, et al. Severe asthma: lessons learned from the National Heart, Lung, and Blood Institute Severe Asthma Research Program. Am J Respir Crit Care Med 2012;185:356–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li X, Hastie AT, Peters MC, Hawkins GA, Phipatanakul W, Li H, et al. Investigation of the relationship between IL-6 and type 2 biomarkers in patients with severe asthma. J Allergy Clin Immunol 2020;145:430–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peters MC, McGrath KW, Hawkins GA, Hastie AT, Levy BD, Israel E, et al. Plasma interleukin-6 concentrations, metabolic dysfunction, and asthma severity: a cross-sectional analysis of two cohorts. Lancet Respir Med 2016;4:574–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hawkins GA, Robinson MB, Hastie AT, Li X, Li H, Moore WC, et al. The IL6R variation Asp(358)Ala is a potential modifier of lung function in subjects with asthma. J Allergy Clin Immunol 2012;130:510–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hastie AT, Moore WC, Li H, Rector BM, Ortega VE, Pascual RM, et al. Biomarker surrogates do not accurately predict sputum eosinophil and neutrophil percentages in asthmatic subjects. J Allergy Clin Immunol 2013;132:72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hastie AT, Mauger DT, Denlinger LC, Coverstone A, Castro M, Erzurum S, et al. Baseline sputum eosinophil + neutrophil subgroups’ clinical characteristics and longitudinal trajectories for NHLBI Severe Asthma Research Program (SARP 3) cohort. J Allergy Clin Immunol 2020;146:222–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 2007;81:559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Denning DW, O’Driscoll BR, Hogaboam CM, Bowyer P, Niven RM. The link between fungi and severe asthma: a summary of the evidence. Eur Respir J 2006;27:615–626. [DOI] [PubMed] [Google Scholar]
- 44.Panettieri RA Jr, Covar R, Grant E, Hillyer EV, Bacharier L. Natural history of asthma: persistence versus progression-does the beginning predict the end? J Allergy Clin Immunol 2008;121:607–613. [DOI] [PubMed] [Google Scholar]
- 45.Ross KR, Gupta R, DeBoer MD, Zein J, Phillips BR, Mauger DT, et al. Severe asthma during childhood and adolescence: A longitudinal study. J Allergy Clin Immunol 2020;145:140–146. [DOI] [PubMed] [Google Scholar]
- 46.Vink NM, Postma DS, Schouten JP, Rosmalen JG, Boezen HM. Gender differences in asthma development and remission during transition through puberty: the TRacking Adolescents’ Individual Lives Survey (TRAILS) study. J Allergy Clin Immunol 2010;126:498–504. [DOI] [PubMed] [Google Scholar]
- 47.Masaki K, Fukunaga K, Matsusaka M, Kabata H, Tanosaki T, Mochimaru T, et al. Characteristics of severe asthma with fungal sensitization. Ann Allergy Asthma Immunol 2017;119:253–257. [DOI] [PubMed] [Google Scholar]
- 48.Li X, Hawkins GA, Ampleford EJ, Moore WC, Li H, Hastie AT, et al. Genome-wide association study identifies TH1 pathway genes associated with lung function in asthmatic patients. J Allergy Clin Immunol 2013;132:313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


