Abstract
Purpose of review:
Atopic dermatitis (AD) and ocular allergy aka Allergic Eye Disease (AED) are two common conditions that often coexist in patients. However, molecular connections between these two conditions are incompletely understood. While common etiologic components including Th2 immune signaling have been suggested for AD and AED, the mechanism how current Th2-targetd therapies (dupilumab, tralokinumab) for AD can augment conjunctivitis is not well understood.
Recent findings:
Differentially regulated genes and pathways relevant for AD disease manifestation are known. In contrast, similar information is not yet available for AED which could be largely addressed by emerging non-invasive ocular sampling techniques. Emerging evidence indicated a reduction in goblet cell number and mucin production in a subpopulation of AD patients with AD leading to adverse ocular outcomes, while other potential mechanisms could also be involved. Involvement of particular barrier function protein(s) in AED needs further investigation.
Summary:
Modern cytokine-targeted therapies for AD showed elevated risk for developing conjunctivitis. Recently developed non-invasive sampling techniques should be leveraged to identify AD endotypes associated with AED and with dupilumab-associated ocular outcomes.
Keywords: Atopic dermatitis, Ocular allergy, biomarker, omics, biologics, barrier dysfunction
1. Introduction
Atopic dermatitis (AD), commonly known as eczema, is a common skin disorder affecting more than 25% children and 10% of adults in the western world.(1) AD is a chronic inflammatory skin condition characterized by dry skin with intense itch, redness, and skin lesions. Although primarily affecting the skin, AD is often associated with a range of systemic manifestations, including ocular diseases. Diseases that affect the ocular surface are very common in patients with AD.(2) Such diseases, including conjunctivitis (allergic and non-allergic), keratitis and blepharitis, are well-known AD-associated comorbidities with combined incidence rates potentially reaching more than 55%.(3) Ocular disease manifestations in AD has previously been described in detail.(4)
Allergic Eye Disease (AED; aka ocular allergy) has been used as an umbrella term which includes allergic conjunctivitis (seasonal and perennial), atopic keratoconjunctivitis, vernal keratoconjunctivitis and giant papillary conjunctivitis; the latter does not show increased lacrimal histamine and considered as iatrogenic.(5) Among these, allergic conjunctivitis alone impacts 6–30% of the general population.(6) AED, which is very commonly reported among AD patients, has been characterized by ocular itch, redness, tearing, and conjunctival swelling leading to decreased productivity negatively impacting the quality of life (QoL).(7, 8) Seasonal allergic conjunctivitis (SAC), perennial allergic conjunctivitis (PAC), Atopic keratoconjunctivitis (AKC) and vernal keratoconjunctivitis (VKC) involve Th2-mediated immune reactions – an etiologic component they share with AD. AED has been managed with antihistamines, mast cell stabilizers, nonsteroidal anti-inflammatory drugs, and steroids.(8) Paradoxically, biologics targeting Th2 cytokine signaling in AD, have shown great promise in treating AD, but demonstrated increased risk for developing AED.(9–13) The mechanism behind this phenomenon is still quite unclear. This has currently elicited significant interest among researchers and clinicians to better understand the etiologic connections between AD and AED. Focusing unique and shared patho-mechanisms between these two conditions is necessary to address these knowledge gaps. This review will present outlines of our current understanding of shared pathways involved in AD and AED, and the most pressing knowledge gaps, such as the unknown etiology of conjunctivitis associated with certain AD-targeted therapies, that needs to be addressed to achieve improved patient care.
2. AED is a significant comorbidity associated with AD and with AD-targeted biologic therapies.
Previous studies have pointed out that among AD patients, rhinitis, asthma and food allergy are very common (approx. 40%, 25%, 24% respectively) comorbidities, whereas allergic conjunctivitis can affect more than 30% of AD patients.(2, 14) Interestingly, patients, when treated with IL-13 and IL-4 signaling blocking antibody Dupilumab (FDA approved for AD) demonstrated significantly increased occurrence of physician-diagnosed AED.(10, 12) These studies reported conjunctivitis as the most common adverse event associated with Dupilumab treatment, particularly in patients with previous history of AED.(3)
The incidence of conjunctivitis secondary to biologic therapy was confirmed from phase three clinical trial of dupilumab where significantly greater number of patients in the treatment arm (treated with dupilumab and topical corticosteroid) showed adverse ocular reactions compared to patients in the control arm (treated with placebo and topical corticosteroids).(15) Of note, conjunctivitis in clinical trials were usually mild which never, or very rarely, required treatment cessation. Therefore, it was not commonly a considered as a very significant question for clinical research. However, since its FDA approval in March 2017, an increasing number of reports of severe dupilumab-induced conjunctivitis have been published. In particular severe dupilumab-induced follicular conjunctivitis without keratitis has been described in a subgroup of patients, which required ophthalmologic intervention.(16) Treatment-associated conjunctivitis has also been reported in case of Tralokinumab and Lebrikizumab, humanized IL-13 blocking antibodies used to treat AD, further indicating a causal link between these treatments and the development of AED.(17, 18) Collectively, biologic-induced eye diseases have gained a greater importance due to steady increase in use of biologics in treating AD and related conditions.
3. Insights from OMICS data obtained using patient derived samples.
3.1. OMICS Data from human AD samples
AD transcriptome has been studied directly from full-thickness skin biopsy samples. Results are publicly available for secondary analysis. Using publicly available multi-origin transcriptomic data we previously described a panel of 89 genes that can be regarded as a general transcriptomic signature for AD.(19) These signature genes were sub-divided into six functional classes: (a) barrier function–related genes, e.g., LCE2B, LOR; (b) chemokine/cytokine genes, e.g., CCL17, CCL18 (c) genes involved in lipid metabolism, e.g., FADS1, FABP7; (d) genes involved in anti-microbial function, e.g., MSMB, LTF (e) protease and protease-inhibitor homeostasis genes, e.g., KLK5, SERPINB3; and (f) genes of diverse metabolic functions, e.g., ARGAP18. In addition to biopsies, much effort has been made to identify transcriptomic biomarkers from minimally invasive skin tape strip samples. More than 12 Genome Wide Association Studies have been done on AD further indicating the involvement of barrier function genes and innate immune genes in AD.(20)
Epidermal dysfunction in AD was also confirmed form proteomic and lipidomic studies. However, skin proteomic studies so far mostly used skin taping to obtain samples. Thus, they were not directly comparable to full-thickness punch biopsy samples. Comparing three independent, stratum corneum proteomic datasets, we previously identified 20 proteins involved in AD pathogenesis.(20) In addition, lipidomic studies also confirmed a dysregulated skin lipid composition(21) which is believed to be driven by Th2 cytokines.(22) Additionally, current data also demonstrated that skin microbiome is different in AD compared to healthy controls, and different in lesional compared to non-lesional skin in AD.(23–27)
Finally, in an attempt to integrate multiple omics layers (e.g., genome, epigenome, transcriptome, proteome, metabolome, lipidome, exposome, microbiome), we identified candidates such as FLG, SPINK5, S100A8, and SERPINB3 present in multiple omic levels that drive AD pathogenesis.(20) Overlapping pathways among different levels include macrophage, endothelial cell and fibroblast activation pathways, and Th1/Th2 and NFkB activation pathways. Interestingly, multi-omics overlaps at the tissue level showed that skin and esophagus were significantly enriched, strongly biological interconnection between AD and food allergy.(20) However, this study was performed in 30 tissue types from the GTEx database which did not include data from ocular cells. Therefore, we were not able to plot the expression of AD-relevant genes in eye samples. Later on, Swamy et al developed a publicly accessible transcriptome database of healthy human eye tissues and a matching web application to query gene expression in eye and other body tissues. (28) This can be a great tool to interrogate eye tissues to find out whether they express AD-relevant genes.
3.1. OMICS results from human EAD samples and therapy-associated adverse ocular outcomes
The pathophysiology of AED has been addressed by several authors. (8, 29) In contrast to accessibility of skin biopsy samples used to identify AD biomarkers, difficulties in obtaining ocular samples and insufficient analytical techniques to analyze ultra-low amount of protein/ RNA samples initially impeded omics studies on ocular allergy. Aydin et al outlined current approaches to identify non-invasive biomarkers of ocular allergy using tear proteomics.(30)
In a recent study involving SAC (N=28), PAC (N = 32) and healthy control (N=35) subjects, Zarzuela and colleagues collected tarsal conjunctival cell samples using brush cytology and analyzed them using flow cytometry which showed major immunologic changes including altered lymphocyte sub-populations associated with allergic conjunctivitis.(31) Matched samples of tear fluid, peripheral blood and ocular microbiome also indicated changes. Omics level studies have not yet been done. Nevertheless, existing data strongly indicates that transcriptomic and proteomic biomarkers can be identified for ocular allergy subtypes facilitating differential diagnosis. Microbiome analysis indicated fungal, and bacterial colonization were observed particularly in the perennial allergic conjunctivitis group. Additional reports about altered ocular microbiome in AD are also available.(4, 32) However, how microbiome influences the ocular immune environment in AD with/ without AED warrants further investigation.
The mechanism(s) for adverse ocular outcomes associated with AD-targeted therapies has not yet been understood completely. Hansen and colleagues showed that IL-13 and IL-4 promote human conjunctival cell proliferation while IFN-γ attenuates proliferation.(33) Since these cells play important roles in maintaining homeostasis of the conjunctival mucosal surface, their changes caused by therapeutic inhibition of IL-4/ IL-13 signaling (e.g. by dupilumab) can provide a mechanistic explanation of dupilumab-associated adverse ocular outcomes. Moreover, this study supports the previous reports confirming the roles of these cytokines in Unfolded Protein Response (UPR) and in changes in mucin production contributing to ocular diseases.(34–36)
Bakker et al examined conjunctival biopsies from six patients suffering from Dupilumab associated conjunctivitis.(37) The authors reported a median density of 3.3 cells/mm of intraepithelial goblet cells in patients receiving dupilumab treatment in contrast to the control groups, who had a median density of 32.3 cells/mm. This was associated with increased infiltration of CD4+ T cells and eosinophils into the conjunctival epithelium. Interestingly, in murine model, ocular IL-13 expression stimulated goblet cell proliferation and mucus secretion.(35) How IL-13 blocking via dupilumab potentially lead to reduction in goblet cell number and mucin production in a subpopulation of patients with AD, warrants investigation. Several other potential mechanisms for therapy-associated adverse ocular outcomes have been proposed, but are yet to be evaluated. Those include upregulated ocular OX40L activity(38), low local concentration of dupilumab drug in the eye,(39) increased IL-17 associated with Demodex mite colonization(13), and most importantly, the significance of transient elevation in circulating eosinophils (40) and basophils in the treatment group warrants further investigation.
4. OMICS results from animal models of AD and AED
4.1. Animal models of AD
Animal models have demonstrated the critical roles of epithelial barrier dysfunction and allergen exposures as important disease-driving factors for AD. Most mice models have been developed by epicutaneous sensitization with allergens or haptens, or by over-expression of cytokines in the skin (reviewed by Jin et al).(41) Th2 skewed immunity induced by perturbagens can dampens the expressions of antimicrobial genes and barrier function genes, which in turn facilitate transcutaneous allergen exposure and altered skin microbiome, and propel disease manifestation. Mouse models have been extensively used to understand the pathomechanism of AD and to test novel therapeutic candidates for this condition. However, individual AD models are often designed to address particular specific research question(s). Therefore the models vary in terms of animal strain(s), genetic background and AD-induction protocols used. This aspect has recently reviewed by Kim and colleagures.(42)
4.2. Animal models of EAD
Similar to AD models, animal models of EAD have been developed based on the sensitization using allergens such as ovalbumin, ragweed pollen, house dust mite, and major cat allergens and subsequent ocular challenges.(43–51) A recent murine model of allergic conjunctivitis demonstrated infiltration of eosinophils into tears and increased antigen-specific IgE titers in tears and the serum.(49) However, as already mentioned, AED denotes a spectrum of conditions that includes SAC, PAC, vernal and atopic keratoconjunctivitis and giant papillary conjunctivitis, with different pathophysiological elements. Existing animal studies mostly utilized murine models mostly focusing allergic conjunctivitis not quite reflecting other conditions.
The role of Th2 signaling in augmenting cutaneous inflammation–associated conjunctivitis was recently shown using mouse model.(52) In this model, mice were exposed to ovalbumin allergen in the presence of MC903 (a vitamin D analog) to induce AD-like skin inflammation. Mice developed severe conjunctivitis when re-exposed to ovalbumin via eye drops. IL-4Rα blockade partially attenuated conjunctivitis manifestation, basophil activation, and Th2 cytokine response. In addition, the results showed differential expression of a group of genes that encode tear proteins (e.g., secretoglobin, lipocalin mucin-like protein family members) associated with ocular disease indicating a potential mechanistic link between IL-4 blockade and ocular outcomes. Several allergens including airborne pollen contain proteases which can disrupt barrier function of the conjunctival intercellular tight junctions augmenting AED. Well established models of ragweed-induced and papain-induced AED have been described.(53) Recent study unraveled the roles of IL-33/ST2/IL-9/IL-9R signaling pathway which can amplify ocular allergic inflammation.(54)
5. OMICS level similarities between AD and AED
Previous studies demonstrated shared etiologic components between AD and EAD. Both conditions are associated with Th2-driven immune reactions. The conjunctiva is usually under prolong exposure to environmental pollutants and allergenic pollens eliciting allergic responses which can lead to IgE-mediated mast cell activation in conjunctival tissue and release of mediators to augment symptoms.(55) Expression of Th2 cytokines and chemokines (VCAM-1, CCL11, CCL24, CCL5, MCP-3, and MCP-4) can lead to infiltration of inflammatory cells (neutrophils, eosinophils, and lymphocytes) to the ocular surface. Several of these immune cells and messengers are also involved in the manifestation of AD symptoms.(4) Furthermore, the potential role of compromised ocular barrier function in AED has been recently emphasized.(56) Of note, epithelial barrier dysfunction has been implicated in several allergic/ inflammatory diseases like AD. However, an elevated expression of barrier function genes (FLG and CLDN-1) on the ocular surface cells of AD patients has been reported, despite dampened cutaneous expression of barrier genes.(57)
Further research is needed to find out whether AD-relevant genes such as 89ADGES member genes are relevant for ocular allergy. To address this knowledge gap, we have investigated the expression of a subset of 89ADGES in ocular tissues using ‘Eye in a Disk’ web application.(28) Our results indicated clustered expression patterns of AD-relevant genes in eye tissue samples (e.g. cornea, retina, lens) at the baseline (figure-1). Capturing changes in gene expression pattern in AED would greatly help in identifying AED biomarkers. This would involve investigations using AED samples to uncover expression changes in ocular tissues due to AED. Given the difficulties in obtaining human ocular samples, developing eye organoid models of AED would greatly accelerate OMICS research on AED.
Figure -1.
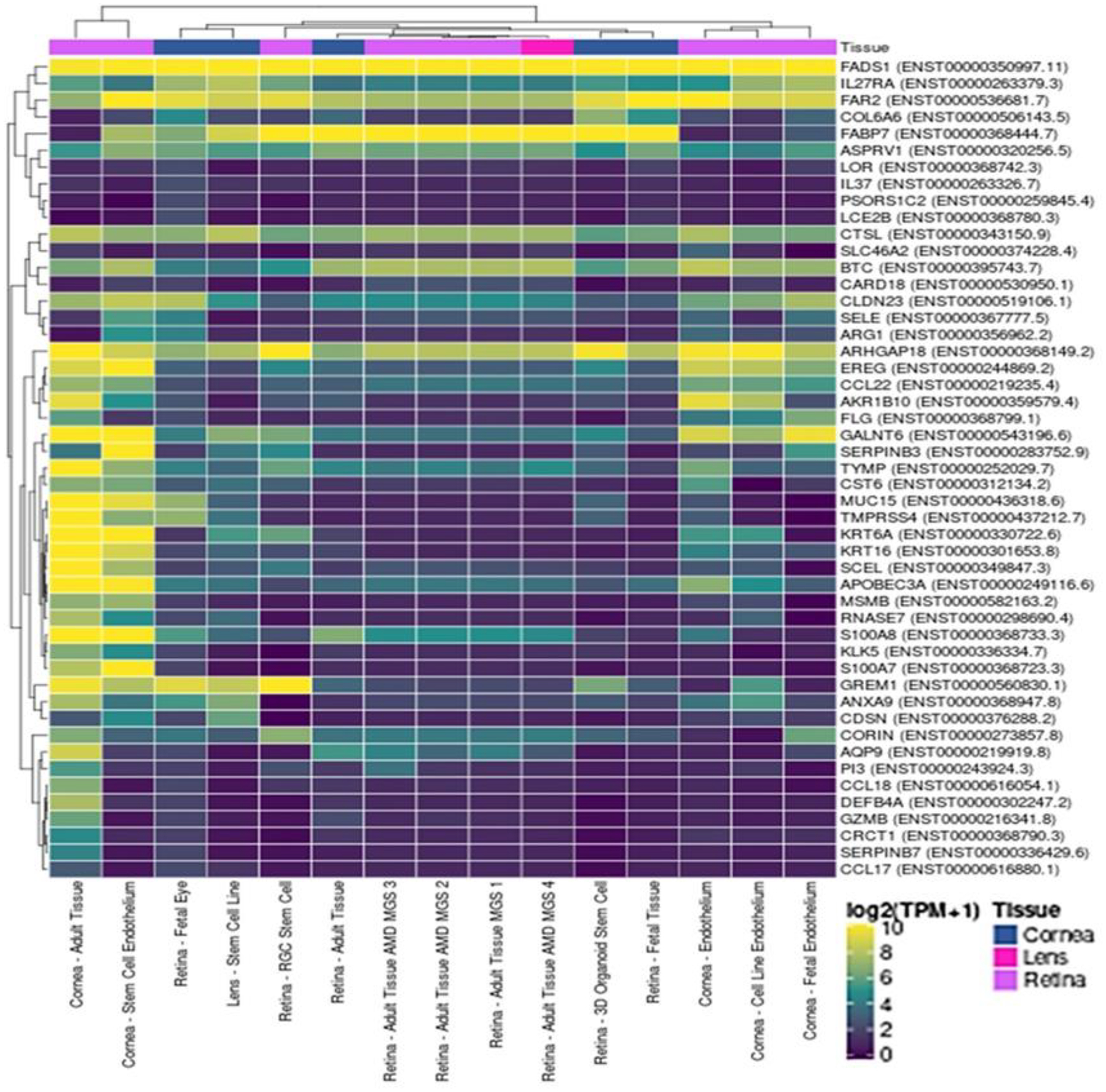
Tissue distribution of 89ADGES member transcripts in the eye. Clustered Heatmap showing abundance of AD-relevant transcripts (vertical axis) in available ocular tissue samples (cornea, retina, lens; horizontal axis). A subset of ADGES (49 top significantly differentiated genes) representing all major functional groups has been used to generate the heatmap. Colors indicate transcript abundance expressed in normalized TPM (transcripts per million) values.
6. Concluding remarks and future directions
Success in omics studies is largely dependent on obtaining samples from well-phenotyped patients, uniform protocol for sample handling, downstream processing and bioinformatic analysis. Identifying endotype-specific biomarkers requires considerable sample size to attain required statistical power. Full-thickness skin biopsies have been traditionally used for OMICS studies and biomarker discovery for AD. This invasive sampling procedure has been a bottleneck for collecting large number of samples, particularly from pediatric patients. However, recent advances in non-invasive/ partially invasive skin sampling using skin tape strips have facilitated omics studies from multiple samples particularly where skin biopsies can be difficult to collect.(58, 59) However, investigators have pointed put significant inter-variability between results, potentially related to differences in sampling and analysis methodologies. Uniform sampling and processing techniques should be used to capture biomarkers relate to AD endotypes and identify endotype(s) particularly associated with allergic eye diseases. In case of AED, difficulties in ocular sample collection have been addressed by other sample collection methods such as cytologic brushing. Additionally, gene expression studies from bulber swab samples have shown great promise indicating that this technique could represent a great non-invasive way of ocular sampling for omics analysis. (60)
Significant progress has been made to understand the mechanisms of AD and EAD. However, there are several pressing questions that need to be addressed. Particularly, (1) which AD endotype is more related to EAD? (2) how biologic therapy against AD can lead to EAD? Further investigation on molecular mechanisms unique to AD and EAD and those shared between these two conditions is urgently needed. Given that biologics are increasingly used to treat AD, and other allergic/ inflammatory diseases, a better understanding about the reduction in intraepithelial goblet cells in biologic treatment-induced conjunctivitis will significantly enhance patient care. (3) Additional research is needed to identify specific subjects at risk for developing treatment-associated conjunctivitis. Dupilumab dosage, AD severity, baseline biomarkers (thymus and activation-regulated chemokine, IgE, eosinophils) and history of conjunctivitis before dupilumab treatment were found to be independent risk factors for dupilumab-induced conjunctivitis.(61, 62) Additionally, why dupilumab-responders showed reduced incidence of conjunctivitis compared to non-responders is not clear.(3) Dupilumab treatment is associated with significant downregulation of AD severity-associate serum biomarkers including thymus-and activation-regulated chemokine (TARC), pulmonary and activation-regulated chemokine (PARC), periostin, and IL-22, and eotaxin-1 and eotaxin-3. The functional roles of these biomarkers in ocular tissue have been incompletely understood.
AED demonstrates several immune abnormalities, some of which are common to multiple allergic/ inflammatory conditions such as AD, while others are unique to AED. The conjunctiva and tears constitute major barrier against environmental insults. In addition to its physical barrier function, it has its own innate immune properties. More research is needed to understand the functions of Conjunctiva Associated Lymphoid Tissue (CALT), to elucidate its connections with the lacrimal component in driving the AED.(31, 63–65) Important data has been reported from ocular samples such as tear. Although informative, results from independent studies could be compared for reproducibility. However, such comparison is currently difficult due to variations in sample types, sample collection, storage and analysis steps.(66) Therefore, uniform optimized protocol for sample handing and downstream cytokine analysis would ensure reproducibility. Additional investigations should involve capturing expression changes of barrier function genes in ocular surface in AED, since enhanced Th2 signaling in AED might disrupt barrier function of the ocular surface. Potential involvement of barrier function genes/ proteins in ocular allergy has recently been emphasized.(56) In this regard, bioengineered organoid models, which greatly facilitated skin barrier function studies, might also be very useful. Cross-disciplinary collaboration between clinicians, molecular biologists and experts in omics would greatly accelerate this effort.
Key points.
Atopic Dermatitis is often associated with allergic eye disease.
Molecular pathways shared between these two conditions are incompletely understood. The current study outlines current ways and understandings to study the common moleculer pathways.
A significant number of patients undergoing biologic therapy for AD (using IL4/IL13 pathway blockade) develops ocular eye disease, the mechanisms of which are not well understood.
We will discuss our current understanding of the mechanisms of this therapy-associated ocular outcomes.
Omics studies on ocular outcomes have been difficult due to difficulties in obtaining ocular samples. We will present outlines of current strategies of obtaining non-invasive skin and eye samples for omics studes.
Financial Support and sponsorship:
This work was supported by the National Institutes of Health (NIH) [NHLBI (R01 HL132344), NHGRI (R01 HG011411), NIAID (R21AI157363)] grants support to TB and by the University of Cincinnati Center for Environmental Genomics Innovator Award (NIH/NIEHS grant number P30ES006096) and University of Cincinnati College of Medicine Interdisciplinary collaborative Challenge Grant to DG.
Footnotes
Conflicts of interest statement: None.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
* Of special interest
** Of outstanding interest
- 1.Cabanillas B, Brehler AC, Novak N. Atopic dermatitis phenotypes and the need for personalized medicine. Curr Opin Allergy Clin Immunol. 2017;17(4):309–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ravn NH, Ahmadzay ZF, Christensen TA, et al. Bidirectional association between atopic dermatitis, conjunctivitis, and other ocular surface diseases: A systematic review and meta-analysis. J Am Acad Dermatol. 2021;85(2):453–61. [DOI] [PubMed] [Google Scholar]
- 3.Akinlade B, Guttman-Yassky E, de Bruin-Weller M, et al. Conjunctivitis in dupilumab clinical trials. Br J Dermatol. 2019;181(3):459–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pietruszynska M, Zawadzka-Krajewska A, Duda P et al. Ophthalmic manifestations of atopic dermatitis. Postepy Dermatol Alergol. 2020;37(2):174–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Middleton’s Allergy Principles and Practices. In: Adkinson NF Jr., Bochner B, Busse WW, Holgate S, Lemanske RF, Simons FER, editors. 1. 7 ed: Mosby Elsevier. [Google Scholar]
- 6.Leonardi A, Castegnaro A, Valerio AL, Lazzarini D. Epidemiology of allergic conjunctivitis: clinical appearance and treatment patterns in a population-based study. Curr Opin Allergy Clin Immunol. 2015;15(5):482–8. [DOI] [PubMed] [Google Scholar]
- 7.Thong BY. Allergic conjunctivitis in Asia. Asia Pac Allergy. 2017;7(2):57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dupuis P, Prokopich CL, Hynes A, Kim H. A contemporary look at allergic conjunctivitis. Allergy Asthma Clin Immunol. 2020;16:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schneeweiss MC, Wyss R, Chin K, et al. Incidence of Bacterial and Nonbacterial Conjunctivitis in Patients With Atopic Dermatitis Treated With Dupilumab: A US Multidatabase Cohort Study. Dermatitis. 2022;33(6S):S73–S82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferreira S, Torres T. Conjunctivitis in patients with atopic dermatitis treated with dupilumab. Drugs Context. 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uchida H, Kamata M, Nagata M, et al. Conjunctivitis in patients with atopic dermatitis treated with dupilumab is associated with higher baseline serum levels of immunoglobulin E and thymus and activation-regulated chemokine but not clinical severity in a real-world setting. J Am Acad Dermatol. 2020;82(5):1247–9. [DOI] [PubMed] [Google Scholar]
- 12.Wollenberg A, Ariens L, Thurau S, et al. Conjunctivitis occurring in atopic dermatitis patients treated with dupilumab-clinical characteristics and treatment. The journal of allergy and clinical immunology In practice. 2018;6(5):1778–80 e1. [DOI] [PubMed] [Google Scholar]
- 13.Thyssen JP. Could conjunctivitis in patients with atopic dermatitis treated with dupilumab be caused by colonization with Demodex and increased interleukin-17 levels? Br J Dermatol. 2018;178(5):1220. [DOI] [PubMed] [Google Scholar]
- 14.Thyssen JP, Halling AS, Schmid-Grendelmeier P et al. Comorbidities of atopic dermatitis-what does the evidence say? The Journal of allergy and clinical immunology. 2023;151(5):1155–62. [DOI] [PubMed] [Google Scholar]
- 15.Blauvelt A, de Bruin-Weller M, Gooderham M, Cather JC, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389(10086):2287–303. [DOI] [PubMed] [Google Scholar]
- 16.Maudinet A, Law-Koune S, Duretz C, et al. Ocular Surface Diseases Induced by Dupilumab in Severe Atopic Dermatitis. Ophthalmol Ther. 2019;8(3):485–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wollenberg A, Beck LA, de Bruin Weller M, et al. Conjunctivitis in adult patients with moderate-to-severe atopic dermatitis: results from five tralokinumab clinical trials. Br J Dermatol. 2022;186(3):453–65. [DOI] [PubMed] [Google Scholar]
- 18.Guttman-Yassky E, Blauvelt A, Eichenfield LF, et al. Efficacy and Safety of Lebrikizumab, a High-Affinity Interleukin 13 Inhibitor, in Adults With Moderate to Severe Atopic Dermatitis: A Phase 2b Randomized Clinical Trial. JAMA Dermatol. 2020;156(4):411–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghosh D, Ding L, Sivaprasad U, et al. Multiple Transcriptome Data Analysis Reveals Biologically Relevant Atopic Dermatitis Signature Genes and Pathways. PloS one. 2015;10(12):e0144316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghosh D, Bernstein JA, Khurana Hershey GK, et al. Leveraging Multilayered “Omics” Data for Atopic Dermatitis: A Road Map to Precision Medicine. Frontiers in immunology. 2018;9:2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bansal A, Simpson EL, Paller AS, Siegfried EC, Blauvelt A, de Bruin-Weller M, et al. Conjunctivitis in Dupilumab Clinical Trials for Adolescents with Atopic Dermatitis or Asthma. Am J Clin Dermatol. 2021;22(1):101–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berdyshev E, Goleva E, Bronova I, et al. Lipid abnormalities in atopic skin are driven by type 2 cytokines. JCI insight. 2018;3(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salava A, Lauerma A. Role of the skin microbiome in atopic dermatitis. Clin Transl Allergy. 2014;4:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams MR, Gallo RL. The role of the skin microbiome in atopic dermatitis. Curr Allergy Asthma Rep. 2015;15(11):65. [DOI] [PubMed] [Google Scholar]
- 25.Chng KR, Tay AS, Li C, et al. Whole metagenome profiling reveals skin microbiome-dependent susceptibility to atopic dermatitis flare. Nat Microbiol. 2016;1(9):16106. [DOI] [PubMed] [Google Scholar]
- 26.Dybboe R, Bandier J, Skov L, et al. The Role of the Skin Microbiome in Atopic Dermatitis: A Systematic Review. Br J Dermatol. 2017. [DOI] [PubMed] [Google Scholar]
- 27.Paller AS, Kong HH, Seed P, et al. The microbiome in patients with atopic dermatitis. The Journal of allergy and clinical immunology. 2019;143(1):26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swamy V, McGaughey D. Eye in a Disk: eyeIntegration Human Pan-Eye and Body Transcriptome Database Version 1.0. Invest Ophthalmol Vis Sci. 2019;60(8):3236–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.La Rosa M, Lionetti E, Reibaldi M, et al. Allergic conjunctivitis: a comprehensive review of the literature. Ital J Pediatr. 2013;39:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aydin E, Dhar P, Gokhale M, et al. A Review of Emerging Tear Proteomics Research on the Ocular Surface in Ocular Allergy. Biology (Basel). 2022;11(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zarzuela JC, Reinoso R, Armentia A, et al. Conjunctival Intraepithelial Lymphocytes, Lacrimal Cytokines and Ocular Commensal Microbiota: Analysis of the Three Main Players in Allergic Conjunctivitis. Frontiers in immunology. 2022;13:911022. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Describes analysis of peripheral blood, conjunctival brush cytology, tear fluid and microbiome samples from healthy controls, and from subjects with seasonal allergic conjunctivitis and perennial allergic conjunctivitis.
- 32.Nakata K, Inoue Y, Harada J, et al. A high incidence of Staphylococcus aureus colonization in the external eyes of patients with atopic dermatitis. Ophthalmology. 2000;107(12):2167–71. [DOI] [PubMed] [Google Scholar]
- 33.Hansen PM, Tollenaere MAX, Hedengran A, et al. IL-4 and IL-13 both contribute to the homeostasis of human conjunctival goblet cells in vitro. Allergy. 2022;77(8):2555–8. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Describes the effects of IL13 signaling in conjunctival goblet cell in relation to biologic therapies for AD involving IL4/IL13 blockage.
- 34.Garcia-Posadas L, Hodges RR, Diebold Y, Dartt DA. Context-Dependent Regulation of Conjunctival Goblet Cell Function by Allergic Mediators. Sci Rep. 2018;8(1):12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tukler Henriksson J, Coursey TG, Corry DB, et al. IL-13 Stimulates Proliferation and Expression of Mucin and Immunomodulatory Genes in Cultured Conjunctival Goblet Cells. Invest Ophthalmol Vis Sci. 2015;56(8):4186–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alam J, de Paiva CS, Pflugfelder SC. Immune - Goblet cell interaction in the conjunctiva. Ocul Surf. 2020;18(2):326–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bakker DS, Ariens LFM, van Luijk C, et al. Goblet cell scarcity and conjunctival inflammation during treatment with dupilumab in patients with atopic dermatitis. Br J Dermatol. 2019;180(5):1248–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mennini M, Dahdah L, Fiocchi A. Two Phase 3 Trials of Dupilumab versus Placebo in Atopic Dermatitis. N Engl J Med. 2017;376(11):1090. [DOI] [PubMed] [Google Scholar]
- 39.Wohlrab J, Werfel T, Wollenberg A. Pathomechanism of dupilumab-associated inflammatory eye symptoms. J Eur Acad Dermatol Venereol. 2019;33(11):e435–e6. [DOI] [PubMed] [Google Scholar]
- 40.Kimura A, Takeda A, Ikebukuro T, Hori J. Serum IgE reduction and paradoxical eosinophilia associated with allergic conjunctivitis after dupilumab therapy. J Ophthalmic Inflamm Infect. 2021;11(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jin H, He R, Oyoshi M, Geha RS. Animal models of atopic dermatitis. The Journal of investigative dermatology. 2009;129(1):31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim D, Kobayashi T, Nagao K. Research Techniques Made Simple: Mouse Models of Atopic Dermatitis. Journal of Investigative Dermatology. 2019;139(5):984–90.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Groneberg DA, Bielory L, Fischer A, et al. Animal models of allergic and inflammatory conjunctivitis. Allergy. 2003;58(11):1101–13. [DOI] [PubMed] [Google Scholar]
- 44.Lee YJ, Han SJ, Lee H, et al. Development of Allergic Conjunctivitis Induced by House Dust Mite Extract From Dermatophagoides pteronyssinus. Invest Ophthalmol Vis Sci. 2016;57(4):1773–81. [DOI] [PubMed] [Google Scholar]
- 45.Cui H, Liu F, Fang Y, Wang T, et al. Neuronal FcepsilonRIalpha directly mediates ocular itch via IgE-immune complex in a mouse model of allergic conjunctivitis. J Neuroinflammation. 2022;19(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]; * mechanism of itch in allergic conjunctivitis.
- 46.Carreras I, Carreras B, McGrath L, Rice A, Easty DL. Activated T cells in an animal model of allergic conjunctivitis. Br J Ophthalmol. 1993;77(8):509–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matsuda A, Hirakata T, Asada Y, Nakae S. Experimental Mouse Models of Ragweed- and Papain-Induced Allergic Conjunctivitis. Methods Mol Biol. 2021;2223:133–49. [DOI] [PubMed] [Google Scholar]
- 48.Mochizuki H, Suyama S, Cha JY, et al. Optimization of a histamine-induced allergic conjunctivitis model in Guinea pigs. J Pharmacol Toxicol Methods. 2022;113:107133. [DOI] [PubMed] [Google Scholar]
- 49.Ogura A, Sugimoto Y. A mouse model of allergic conjunctivitis permitting tear eosinophil quantification. J Pharmacol Toxicol Methods. 2022;118:107225. [DOI] [PubMed] [Google Scholar]
- 50.Tang YJ, Chang HH, Chiang CY, et al. A Murine Model of Acute Allergic Conjunctivitis Induced by Continuous Exposure to Particulate Matter 2.5. Invest Ophthalmol Vis Sci. 2019;60(6):2118–26. [DOI] [PubMed] [Google Scholar]
- 51.Giavina-Bianchi P, Kalil J, Rizzo LV. Development of an animal model for allergic conjunctivitis: influence of genetic factors and allergen concentration on immune response. Acta Ophthalmol. 2008;86(6):670–5. [DOI] [PubMed] [Google Scholar]
- 52.Han H, Cummings S, Shade KC, et al. Cellular mechanisms and effects of IL-4 receptor blockade in experimental conjunctivitis evoked by skin inflammation. JCI insight. 2023;8(3). [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Mechanistic study on IL4 signaling blockade in the context of cutaneous inflammation associated experimental conjunctivitis.
- 53.Matsuda A, Hirakata T, Asada Y, Nakae S. Experimental Mouse Models of Ragweed- and Papain-Induced Allergic Conjunctivitis. In: Nagamoto-Combs K, editor. Animal Models of Allergic Disease: Methods and Protocols. New York, NY: Springer US; 2021. p. 133–49. [DOI] [PubMed] [Google Scholar]
- 54.Hu J, Gao N, Zhang Y, et al. IL-33/ST2/IL-9/IL-9R signaling disrupts ocular surface barrier in allergic inflammation. Mucosal Immunol. 2020;13(6):919–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Elieh Ali Komi D, Rambasek T, Bielory L. Clinical implications of mast cell involvement in allergic conjunctivitis. Allergy. 2018;73(3):528–39. [DOI] [PubMed] [Google Scholar]
- 56.Singh N, Diebold Y, Sahu SK, Leonardi A. Epithelial barrier dysfunction in ocular allergy. Allergy. 2022;77(5):1360–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Callou TMP, Orfali RL, Sotto MN, et al. Increased expression of Filaggrin and Claudin-1 in the ocular surface of patients with atopic dermatitis. J Eur Acad Dermatol Venereol. 2022;36(2):247–54. [DOI] [PubMed] [Google Scholar]; * Describes increased expression of barrier function genes in the ocular surface of AD subjects.
- 58.He H, Bissonnette R, Wu J, et al. Tape strips detect distinct immune and barrier profiles in atopic dermatitis and psoriasis. The Journal of allergy and clinical immunology. 2021;147(1):199–212. [DOI] [PubMed] [Google Scholar]
- 59.Hu T, Todberg T, Andersen D, et al. Profiling the Atopic Dermatitis Epidermal Transcriptome by Tape Stripping and BRB-seq. International journal of molecular sciences. 2022;23(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alenezi H, Ozkan J, Willcox M, et al. Differential gene expression of the healthy conjunctiva during the day. Cont Lens Anterior Eye. 2022;45(4):101494. [DOI] [PubMed] [Google Scholar]; * Utility of bulber swab sampling in gene expression studies.
- 61.Agnihotri G, Shi K, Lio PA. A Clinician’s Guide to the Recognition and Management of Dupilumab-Associated Conjunctivitis. Drugs R D. 2019;19(4):311–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Treister AD, Kraff-Cooper C, Lio PA. Risk Factors for Dupilumab-Associated Conjunctivitis in Patients With Atopic Dermatitis. JAMA Dermatol. 2018;154(10):1208–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Steven P, Schwab S, Kiesewetter A, et al. Disease-Specific Expression of Conjunctiva Associated Lymphoid Tissue (CALT) in Mouse Models of Dry Eye Disease and Ocular Allergy. International journal of molecular sciences. 2020;21(20). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Siebelmann S, Gehlsen U, Huttmann G, et al. Development, alteration and real time dynamics of conjunctiva-associated lymphoid tissue. PloS one. 2013;8(12):e82355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Steven P, Gebert A. Conjunctiva-associated lymphoid tissue - current knowledge, animal models and experimental prospects. Ophthalmic Res. 2009;42(1):2–8. [DOI] [PubMed] [Google Scholar]
- 66.Ghosh D, Mersha TB. Publicly available cytokine data: Limitations and opportunities. The Journal of allergy and clinical immunology. 2022;150(5):1053–6. [DOI] [PubMed] [Google Scholar]


