Abstract
The Many Hosts of Mycobacteria (MHM) meeting series brings together basic scientists, clinicians and veterinarians to promote robust discussion and dissemination of recent advances in our knowledge of numerous mycobacterial diseases, including human and bovine tuberculosis (TB), nontuberculous mycobacteria (NTM) infection, Hansen’s disease (leprosy), Buruli ulcer and Johne’s disease. The 9th MHM conference (MHM9) was held in July 2022 at The Ohio State University (OSU) and centered around the theme of “Confounders of Mycobacterial Disease.” Confounders can and often do drive the transmission of mycobacterial diseases, as well as impact surveillance and treatment outcomes. Various confounders were presented and discussed at MHM9 including those that originate from the host (comorbidities and coinfections) as well as those arising from the environment (e.g., zoonotic exposures), economic inequality (e.g. healthcare disparities), stigma (a confounder of leprosy and TB for millennia), and historical neglect (a confounder in Native American Nations). This conference report summarizes select talks given at MHM9 highlighting recent research advances, as well as talks regarding the historic and ongoing impact of TB and other infectious diseases on Native American Nations, including those in Southwestern Alaska where the regional TB incidence rate is among the highest in the Western hemisphere.
I. INTRODUCTION
Mycobacteria comprise obligate and opportunistic pathogens that infect a wide range of hosts, including humans, wildlife and livestock. Infections can either be asymptomatic or lead to clinical diseases which vary widely in presentation depending on the species. The diseases caused by mycobacteria occur in nearly every country, disproportionately affecting communities that are already challenged by health inequalities. Mycobacterial disease monitoring and treatment efforts were severely hampered by the Coronavirus Disease 2019 (COVID-19, or COVID) pandemic. The challenges associated with preventing and controlling mycobacterial diseases may at times feel insurmountable to scientists and clinicians; however, the rapidity with which COVID-associated research data were shared and effective vaccines were developed is proof that institutions, scientists and clinicians—when motivated and given sufficient resources—can collectively overcome seemingly insurmountable challenges.
Since no single institution can address all mycobacterial diseases and their accompanying challenges, the Many Hosts of Mycobacteria (MHM) conference series has since 2007 brought together mycobacteria experts from around the globe for presentations and discussions on the latest developments in basic, translational and clinical mycobacteria research. The 9th Many Hosts of Mycobacteria conference (MHM9) was held at The Ohio State University (OSU) in Columbus, OH, July 11–13, 2022. MHM9 was attended by ~70 individuals, via both in person and remote attendance options. The individuals presenting and attending represented multiple communities, tribes and nations. The three-day MHM9 conference was divided into several sessions, the themes and proceedings of which are summarized below and outlined in TABLE 1.
TABLE 1.
MHM9 sessions and select associated Presentations
| I. | Round table introductions |
| II. | Infectious diseases in Indigenous health |
| II-a. The history of tuberculosis among Native Nations | |
| II-b. COVID-19 in tribal communities | |
| II-c. Vaccine development to target STIs in Native American communities | |
| II-d. Impact of host environment on Mtb fitness and drug efficacy | |
| III. | Tuberculosis in Alaska |
| III-a. The introduction of TB by explorers and colonists | |
| III-b. Effects of TB sanitoria on Alaskan Natives | |
| III-c. TB chemotherapy studies on Alaskan Natives | |
| III-d. Uneven geographic distribution of TB in Alaska | |
| III-e. Hurdles in the path to reducing TB rates in Alaskan Native communities | |
| IV. | Mycobacteria in the soil, water, and wild |
| IV-a. Environmental NTM hotspots of the Hawaiian islands | |
| IV-b. Buruli ulcer and M. ulcerans susceptibility to Telacebec (Q203) | |
| IV-c. Johne’s disease and M. paratuberculosis | |
| IV-d. M. bovis infections in wild carnivores | |
| V. | Mycobacterial disease comorbidities and confounders |
| V-a. M. abscessus and cystic fibrosis | |
| V-b. Adipocyte dysfunction, insulin resistance in TB | |
| V-c. Metabolic comorbidities and TB | |
| V-d. M. avium infection and cardiac dysfunction | |
| V-e. NTM and COPD | |
| VI. | Models for assessing mycobacterial disease and coinfections |
| VI-a. Modeling the M. tuberculosis carriage state | |
| VI-b. Biomarker utility in the guinea pig infection model of TB | |
| VI-c. Humanized mice and HIV/Mtb coinfection | |
| VI-d. Rabbit model of TB latency and reactivation for HDT studies | |
| VI-e. Epigenetic modifications in BCG-vaccinated infants | |
| VI-f. Leprosy and soil-transmitted helminths | |
| VII. | Leveraging the lessons of mycobacteria to improve health |
| VII-a. Mycobacteria and lung transplantation | |
| VII-b. Battling water laden NTM with UV emitting diodes | |
| VII-c. Old friends: stress resilience properties of M. vaccae NCTC 11659 | |
| VII-d. Sterilizing immunity following i.v. vaccination with BCG | |
| VIII. | Leprosy and its associated disparities |
| VIII-a. Leprosy in Norway in the 19th & 20th centuries | |
| VIII-b. Leprosy in modern Brazil | |
| VIII-c. Leprosy in modern US | |
| VIII-d. Clinical experiences treating leprosy in US, Mexico, Colombia & Cuba | |
| VIII-e. Armadillo model for leprosy neuropathy | |
| VIII-f. Blood transcriptome in leprosy reversal reaction | |
| IX. | Bovine tuberculosis and its environmental confounders |
| IX-a. M. bovis genomic signatures across organizational scales | |
| IX-b. Bovine TB in badgers | |
| IX-c. Oral vaccination against bovine TB for free-ranging white-tailed deer | |
| IX-d. World M. bovis project | |
| IX-e. Zoonotic M. bovis disease following exposure to wild deer in Michigan | |
| X. | Recent innovations in mycobacterial research |
| X-a. Mouse model of post-primary pulmonary TB | |
| X-b. Artificial intelligence assisted histological analysis | |
| X-c. Exploiting M. smegmatis to understand mycobacterial pathogenesis | |
| X-d. Mycobacteria evolution within the granuloma | |
| XI. | Remembering those who have passed |
II. INFECTIOUS DISEASES IN INDIGENOUS HEALTH
Mycobacterial diseases and their associated coinfections/comorbidities disproportionately impact Native American communities 1–3. MHM9 Day 1 comprised of a workshop focused on the specific impact of tuberculosis (TB) and other infectious diseases in American Indian/Alaskan Native (AI/AN) communities from the initial phases of colonization to the present, their intersection with dispossession and disenfranchisement, and the links between infectious disease and other prominent health conditions of special interest to Indigenous communities (e.g., cardiovascular disease, diabetes). The workshop was led entirely by Indigenous persons with local and national expertise in infectious disease and its intersection with Native American social and historical contexts, with presentations by individuals across all scientific career stages, from the following tribes and nations: Shawnee, Eastern Band of Cherokee Indians, Luiseño and Wailaki, Nakoda, Seneca, Zuni Pueblo and Mescalero Apache, Bad River, Diné, and Taos Pueblo. Invited speakers and panelists included Dr. Matthew Anderson (OSU), Madison Eagle, LSW (OSU), Dr. Elizabeth Nelson (Pasteur Institute), Dr. Temet McMichael (previously Centers for Disease Control and Prevention), medical student Emily Plumage (OSU), Dr. Naomi Lee (Northern Arizona University), PhD candidate Saydie Sago (University of Colorado–Boulder), Dr. Elise Lamont (University of Minnesota), Dr. Krystal Tsosie (Arizona State University), undergraduate student Aislinn Concha (University of Colorado–Boulder), and PhD candidate Caelan Wright (University of Colorado–Boulder). Summaries of select talks are provided below.
II-a. The History of TB Among Native Nations.
Throughout the history of Native Nations, the spread of infectious disease has been driven by intertwined social, cultural and biological forces. TB is a prime example of this bio-cultural phenomenon where introduction, dissemination, and endemicity have been linked to socio-political and cultural events 4–7. To elucidate those factors that have influenced the evolution and transmission of the Mycobacterium tuberculosis complex (MTBC) across Native nations, Dr. Nelson, with her coauthors, performs synergistic analyses of modern and ancient genomic data in combination with archeological information, historical records and documents. In the Eastern Hemisphere, records from the physician Hippocrates (BCE 460-375) contain some of the earliest mentions of TB. In the Western Hemisphere, the earliest convincing cases of skeletal TB identified trace back to 290-700 CE in South America, and 900 CE in North America 8–10. By the Late Intermediate Period of the Andes (circa 1000-1400 CE), the abundance of skeletal signs consistent with TB in South America, with several identified cases in North American contexts, suggests MTBC was circulating in populations across the American continents 8.
Skeletal evidence from South America has been complemented with the paleogenomic identification of pre-colonial strains of M. pinnipedii, a member of the animal clade of MTBC, causing human TB in ancient populations of coastal and inland Peru and mountainous regions of Colombia 11, 12. The identification of this pathogen across this diverse ecological landscape, along with the broad host tropism of the animal clade 13 suggests a variety of hosts and likely many mycobacterial species were facilitating the dissemination of this disease in ancient Andean contexts. Although current archaeological and paleogenomic evidence suggests pre-European strains were widely present in the Americas, modern data suggests that the arrival of Europeans triggered the replacement of animal MTBC strains as the dominant agents of human infection with the European lineage, L4 14. As one of the most virulent and clinically concerning M. tuberculosis lineages, it believed the introduction of L4 contributed significant morbidity and mortality in history, across both the European and American continents.
In North America, TB took an especially large toll on displaced First Nations and Native groups who were pushed from their land with western migrations of Europeans. This is particularly striking in Native children who were taken from their families and sent to boarding schools through assimilationist government programs 15, 16. Historic reports show TB was a leading cause of death in several Indigenous communities during the 19th to 20th centuries 17–20. Dr. Nelson and colleagues highlighted differences in treatment and access to care between the Settler and Native experience that they say directly lead to the health inequality we see today, and reflected on perseverance and resilience of the notable figure Dr. Susan La Flesche Picotte 21. To conclude her presentation, Dr. Nelson noted that the often-used term “pandemics of the past”, is inaccurate and falls short to recognize the long-lasting impacts of diseases, such as TB, that were spread and propagated under colonial systems and the disruption of traditional ways. The history of structural violence acted out by colonial structures and practices that exploited and harmed Native Nations must be acknowledged, disrupted, and replaced with Indigenous-centered and historically aware medical and research practices that respect Native culture and sovereignty.
II-b. COVID-19 in Tribal Communities.
There are currently 574 federally recognized tribes within the United States (US) and each maintains a government-to-government relationship with the US government. Each American Indian and Alaska Native (AI/AN) Tribal Nation may collectively refer to themselves as tribes, Tribal Nations, bands, pueblos, communities, villages, or other names. It is important to understand these terms can differ based on individual or tribal preferences, but in either case, it is important to address tribes and their peoples based on their preferences. Tribal healthcare provided to AI/ANs is delivered by a part of the federal government called the Indian Health Services (IHS) which provides for the overall health and wellbeing of AI/ANs based on obligations codified in treaties made by the federal government. Using federal funds, tribes can also form organizations or health centers, often called 638 Facilities, which are authorized under the Indian Self-Determination and Education Assistance Act of 1975. In some circumstances, multiple tribes may band together and form consortiums to better meet the needs of their respective peoples. It is important to acknowledge that these relationships and decisions are derived from each tribe’s sovereignty, and it is the responsibility of the US government to provide for the medical and healthcare needs of AI/ANs based on treaty law. It is also important to note that although the health and wellbeing of AI/ANs is the primary role of the IHS, there are many other advocates and key federal agencies that share in this responsibility. This includes but is not limited to the Bureau of Indian Affairs (BIA), the Centers for Disease Control and Prevention (CDC), Tribal Epidemiology Centers (TECs), and State and local Public Health Departments.
Dr. McMichael spoke of his experiences at the beginning of the COVID-19 pandemic while stationed at Public Health – Seattle & King County and his involvement with CDC’s Tribal Support Section (TSS) which was pioneered by key advocates and leadership at CDC to meet the needs of AI/ANs as a result of the COVID-19 pandemic. McMichael also recalls being deployed to a Tribal Nation in April 2020, shortly after his involvement in the initial response in Seattle and King County. He elaborated on the complex history shared by many Tribal Nations, the US government and its healthcare agencies, and emphasized that although modern medicine has explicit roles in tribal communities—traditional healing and spiritual medicine are also interwoven into AI/AN cultures.
In challenging times like the COVID-19 pandemic, it is important to utilize modern medicine while acknowledging the importance of traditional practices. McMichael then focused on public health at the local level, describing the composition of the County of San Diego Tribal Nations, which has 17 federally recognized tribes within its modern borders. He noted that these modern borders do not necessarily align with traditional territories, which can make public health response activities and jurisdictional considerations challenging. He notes that many San Diego County tribes formed consortiums with their local IHS partners and appointed Public Health Authorities (PHAs) within each tribe to address needs during the pandemic. These PHAs were appointed by the tribes to receive their public health information from County of San Diego’s Health Department, and in accordance with tribal sovereignty take the action deemed appropriate by each Tribal Nation. This system is important because it empowers Tribes to own their data and to use their data to make decisions in accordance with Tribal sovereignty.
Key takeaways from Dr. McMichael’s presentation were that Tribal communities have been and still are underserved, are impacted by gaps in public health data, and are disproportionately affected by COVID-19. When considering public health measures, it is necessary to understand the structure of Tribes, their sovereignty, and to be receptive to Tribal needs. He also notes that despite these hurdles, there were and are many key advocates and leadership who mobilized to meet the needs of AI/ANs during the pandemic and commends those efforts. He feels honored to have played the role(s) he did in that process and strongly believes that addressing these inequities will be driven by empowering key personnel both internally and externally at federal, state, and local levels.
II-c. Vaccine Development to Target STIs in Native American Communities.
TB is not the only disease which disproportionately affects Native American communities. Human papillomavirus (HPV) is the most common sexually transmitted infection (STI) in the US. There are >30 circulating HPV genotypes, some of which are low-risk and cause warts, while other high-risk HPV (hrHPV) genotypes cause cervical cancer, as well as cancers of the penis, anus, head and neck. Dr. Lee spoke of her work sequencing prevalent HPV genotypes and assessing the vaginal microbiome of Native American women. An important result of this work 22, which was the largest study of HPV prevalence in Native American women conducted to date, was that the combined prevalence of 14 hrHPV genotypes in her partner Tribal community was higher than the US national average of all similarly aged women (34.8% vs 20.7%). The data further indicated that as age increases, hrHPV prevalence decreases while cervical cancers increase, and that women who were older at the time of infection have a more difficult time clearing the infection. Dr. Lee is now working with additional Native Nations in Arizona to establish hrHPV genotype prevalence across age and other demographic criteria. Importantly, it was noted that although HPV is vaccine-preventable, Native populations have been susceptible to misconceptions about the HPV vaccine, including the false belief that the HPV vaccine is a treatment (HPV vaccination prevents infection, and is not a therapy or treatment for existing HPV infection) 23. Furthermore, the existing HPV vaccine targets viral antigens (i.e., the HPV L1 protein) expressed by HPV genotypes that are common in the US population as a whole, but less common in the Tribal communities she works with; therefore, to more effectively prevent HPV infections in American Indians, it would be advantageous for next generation HPV vaccines to target the L1 and L2 protein sequences prevalent in Native Nations 24. Dr. Lee concluded by sharing a Lakota prayer that inspires her and others in the Native American vaccine research and development space: “Do this so that the people may live.”
II-d. Impact of the host environment on Mtb fitness and drug efficacy.
The mechanisms of antibiotics and chemotherapeutics have been defined by the isolated interactions of drugs with their cognate targets. The untethering of the pharmacology of antibiotics from the host immune response has limited our understanding of how antibiotics function in vivo, especially regarding diseases that manifest a spectrum of outcomes. TB encompasses several disease trajectories including bacterial clearance, latency, reactivation, and death. Likely factors impacting TB outcome as well as anti-TB drug efficacy are the lung microenvironment, host response, and immune status. Pyrazinamide (PZA), a cornerstone of the first-line therapy for TB, shows potent in vivo activity but is limited under traditional in vitro conditions. While the bacterial target of PZA remains unknown, emerging evidence demonstrates a previously unrecognized role of the host stress response in PZA activity. Dr. Lamont showed that interferons capable of modulating the macrophage environment are critical for PZA function in vivo using mouse models of TB. Mice deficient for interferon responses showed unrestricted growth of M. tuberculosis in secondary lymphoid organs despite PZA treatment. Recent studies have reported that the lung microbiome influences and trains local immune responses. There is a paucity of data examining the contribution of the lung microbiome in TB susceptibility, progression, and anti-TB drug activity. Using a mouse model that produces various TB lesions coupled with naturalization of the microbiome (i.e. the introduction of commensal microbes to an otherwise sterile mucosal site), Dr. Lamont demonstrated a significant change in M. tuberculosis burden in the lung when compared to specific pathogen free (SPF) counterparts. Immune response profiling of the lungs from naturalized microbiome mice showed alterations in protective factors indicative of M. tuberculosis control compared to SPF mice. Future studies will explore the role of the microbiome in anti-TB drug therapy. Together these data suggest a critical role of the host immune response in anti-TB drug mechanisms of action and Mtb growth. Unifying antibiotic pharmacology with the host immune response will aid in our understanding of drug treatment failure in certain subsets of patients as well as directing future anti-TB drug design.
III. TUBERCULOSIS IN ALASKA
There is a tendency within the US to perceive TB as a disease which only afflicts foreign lands 25, 26. For Americans in Alaska, however, the perception of TB as a foreign disease does not match their past or present reality, as TB incidence in Northern and Southwestern regions of Alaska rival those of so-called “TB endemic” countries (FIG 1, 2). To provide a history of TB in Alaska and share their perspectives on the reasons why TB incidence remains higher than any other state in the US, Dr. Bruce Chandler (Alaska Division of Public Health), Dr. Michelle Rothoff (Alaska Division of Public Health) and Dr. Timothy Lemaire (Norton Sound Health Corporation) presented remotely from their respective duty stations in Anchorage and Nome, AK. Key takeaways from their presentations are as follows:
FIGURE 1. Tuberculosis among Americans living in Alaska.
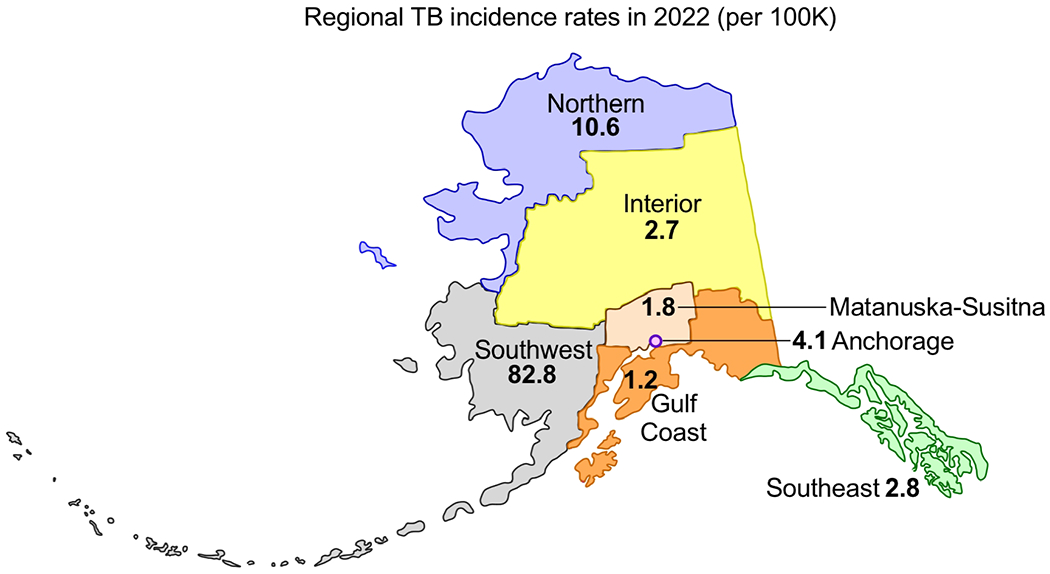
The geographical distribution of TB incidence in the state of Alaska for the year 2022, as reported by the Alaska Department of Health for the Northern, Interior, Southwest, Matanuska-Susitna, Gulf Coast and Southeast regions, as well as the capital city Anchorage.
FIGURE 2. The TB incidence rate of Northern and Southwest Alaska relative to all countries in the Western Hemisphere.
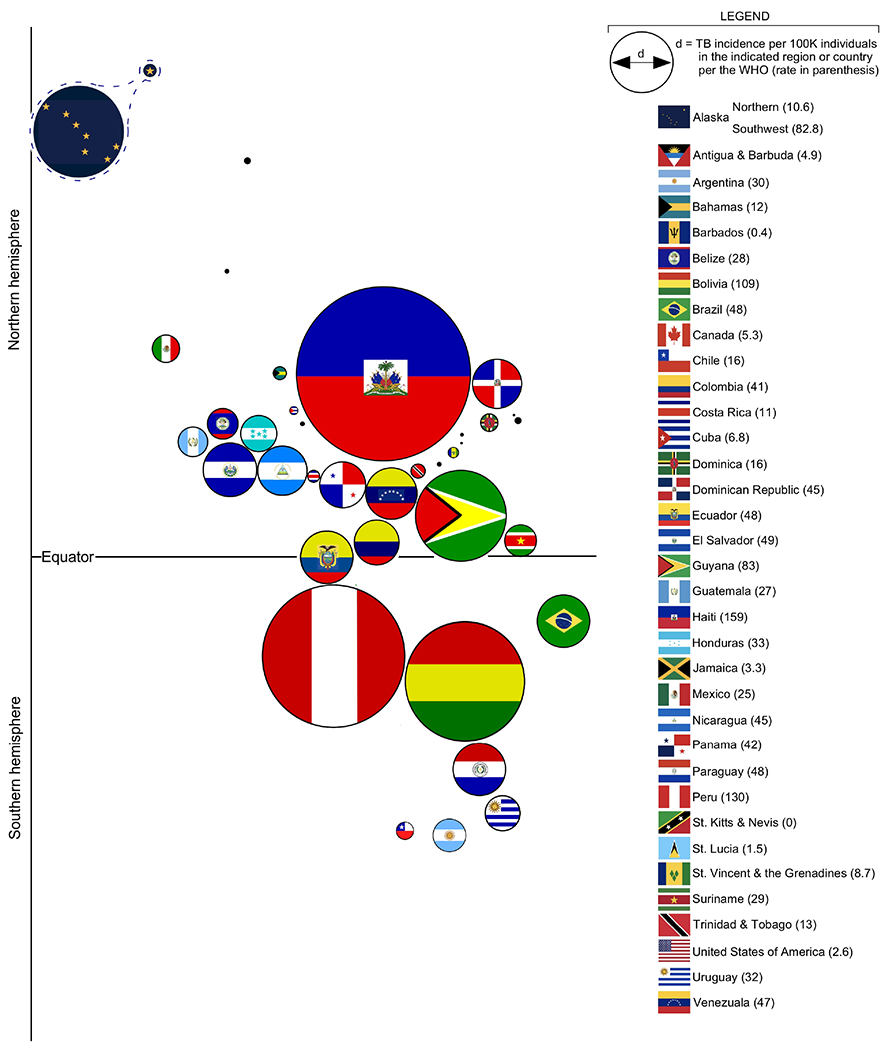
Bubble chart depiction of TB incidence in each country of the Western Hemisphere, per 2021 data reported by the World Health Organization. The diameter of each bubble is directly proportional to TB incidence in the indicated country, each country being represented by its flag. Bubbles are generally organized according to the latitude and longitude coordinates of the corresponding country’s capital city. For comparison purposes, the incidence rates of Northern and Southwest Alaska--the bubble for which are represented by Alaska flag components--are treated separately from all other US states. Note that any country with an incidence of 5/100K or below is depicted by a black dot.
III-a. Explorers and colonists introduced TB to Alaskan Natives.
Although Alaskan Natives have been present for millennia 27, it was not until more recently (historically speaking) that human-adapted TB came to Alaska, with the arrival of Russian explorers in the 1700s. Russian explorers were among the first settlers to arrive in Alaska in search of wealth and resources, bringing with them TB and other infectious diseases such as measles, mumps, influenza, polio, smallpox, and syphilis. In time, so too would colonists from Europe and the newly formed United States of America (USA) bring TB from areas where the disease was widespread, in their pursuit of fur, gold and whales.
III-b. TB and sanitoria practices had devastating effects on Alaskan Natives and their culture.
It is difficult for non-Indigenous people to grasp the devastation TB caused in Alaska before effective TB chemotherapy became available in the 1950s, both in terms of loss of life and loss of culture. During the 1800s and early 1900s, TB rapidly spread among Alaskan Native (AN) people, a susceptible population living under conditions ideal for TB transmission and dissemination, especially for those with close contact to the incoming colonists. Many of these factors continue to sustain TB in Alaska, especially among ANs in northern and southwestern Alaska (FIG 1), where TB incidence rates exceed most other countries in the Western Hemisphere (FIG 2). Among AN children, surveys conducted from 1948–1951 found that TB was most serious along the lower Yukon and Kuskokwim Rivers in western Alaska, where 75% of Alaskan Native children aged <8 years (and 92% of those aged 7–8 years) had positive tuberculin skin tests 28. For comparison, the tuberculin positivity among White children in Alaska at that time was 0.6-6% 28 In other regions, 32% of AN children in Southeast Alaska and the Aleutian Islands and 56% of children in the Interior region were found to be infected with TB 28. For ANs, the loss caused by TB was compounded by practices at the sanitaria which were established in the 1940s to care for AN adults and children with TB. Many never returned from these sanitaria and their families never received any news of what had happened to them. Many remain in a mass internment site in Sitka, Alaska (FIG 3).
FIGURE 3. A mausoleum for unknown and unclaimed victims of TB in Sitka, Alaska.

Photo of a World War II ammunition bunker, at Mt. Edgecumbe in Sitka, which was repurposed to be a mausoleum for deceased TB patients in the 1940s and 1950s. No funds were available to transport remains back to their loved ones for half a century. Fifty years later, if relatives could be located, some were transported home for burial. The others remained buried here in Sitka. Image courtesy of Bruce Chandler.
III-c. TB chemotherapy studies on Alaskan Natives informed the current US TB control strategy.
As the Alaska statehood movement was gaining momentum during the 1950s, so too did this decade bring about big changes to TB in Alaska, as the severity of Alaska’s TB epidemic was recognized by federal and territorial agencies, effective chemotherapy became available, and a coordinated federal and territorial campaign to control TB was started 29, 30. Five elements of that campaign are as follows: (1) the creation of the 400-bed Alaskan Native Hospital in Anchorage, the 200-bed Mt. Edgecumbe Hospital in Sitka and regional hospitals and clinics throughout the state; (2) the creation of the Alaskan Native health care system and Alaska’s public health system; (3) groundbreaking studies on controlling and preventing TB were conducted in Alaska, including the original demonstration of isoniazid’s effectiveness and on use of isoniazid prophylaxis to prevent active TB in the Yukon-Kuskokwim (YK) delta 31, 32; (4) The Ambulatory Chemotherapy Program started in January 1955 in the Bethel service area in western Alaska and soon expanded to villages in northern and central Alaska. The program demonstrated that many people with active TB could be treated and cured without being hospitalized. As a result of these studies on ANs with TB, ambulatory outpatient chemotherapy became accepted for treatment of people with active disease in the US, and is still part of the US TB control strategy.
III-d. The geographic distribution of TB in Alaska is uneven, the Southwest regional rate being among the highest in the Western Hemisphere.
Despite the progress which started in the 1950s, the rates of active TB remain significantly higher in the northern region (which includes the north slope and Nome) and especially in the Southwest region (which includes Bethel, Dillingham, Bristol Bay, and the Aleutians) (FIG 1). These two regions of the state have historically held Alaska’s (AK’s) highest rates of TB and ongoing outbreaks in villages in these regions continue to pose an ongoing challenge to TB control in AK. AK’s Northern and SW regions have rates that are among the highest in the Western Hemisphere. There are multiple reasons for this: the historical TB outbreaks described above were never fully extinguished, ongoing challenges with living conditions, barriers to care and geographical isolation. Prior to 2020, there had been a trend toward a slow decline in TB rates at both the state and national level; however, this halted or reversed temporarily as a result of the COVID pandemic. While the US as a whole had a 20% decrease in the TB incidence rate from 2019-2020 (likely a result of underdiagnosis/underreporting related to COVID), the AK TB incidence rate remained the same during that period, ~2×-3× times higher than the national rate 33. Current trends in case numbers at the time of MHM9 indicated the 2022 rate would likely be higher than 2021; this bore out in the eventual published AK data 33.
III-e. North to the future: hurdles in the path to reducing NA/AN TB rates.
Perennial challenges to public health efforts to reduce TB in AK stem in large part from the fact that 82% of AK’s communities (including those most impacted by TB) are off the road system. As such, the process by which active TB is detected can be tediously slow in rural and often roadless Alaska. Several hundred air or boat miles can separate a patient from a chest x-ray and/or sputum collection. Additionally, the only TB reference lab in the state is many hundreds of air miles away albeit centrally located in Anchorage. Under such conditions, serial acid fast bacillus (AFB) sputum samples may take up to a week to process. This can result in either delayed onset of treatment or unnecessary empiric treatment calculated to both treat the patient and protect the community. This dilemma often results in precautionary hospital isolation of a patient who might otherwise be able to travel home by air. It has also been noted that medical doctors and other providers coming to Alaska to practice or for their residency training are often unprepared for identifying TB. Finally, and perhaps the most challenging hurdle of all for non-Indigenous scientists wishing to research the factors contributing to TB transmission amongst NA/ANs, variable levels of distrust of non-Indigenous healthcare workers and systems exist among the NA/AN Nations most affected by TB because of historical mistreatment through health systems and more broadly via forced relocations from ancestral territories and efforts to extinguish cultural practices.
IV. MYCOBACTERIA IN THE SOIL, WATER, AND WILD
The Mycobacterium genus includes non-pathogenic and opportunistic species which are found in both natural and engineered environments 34, 35, as well as obligate pathogens which cause chronic infectious diseases in wild animals, livestock and humans. To address the breadth of environments and hosts which can harbor mycobacteria, Dr. Jennifer Honda (University of Texas-Tyler), Dr. Paul Converse (Johns Hopkins), Dr. Ray Ball (Eckerd College), Dr. Michele Miller (Stellenbosch University), Dr. Michelle Larsen (Albert Einstein College of Medicine), and Dr. Kathleen Alexander (Virginia Tech) respectively spoke on the subjects of environmental nontuberculous mycobacteria (NTM) hot spots in the Hawaiian Islands, M. ulcerans and Buruli ulcer, M. avium ss. paratuberculosis and Johne’s Disease, M. bovis infections among wild carnivores of Kruger National Park (South Africa), novel animal-adapted mycobacteria, and M. mungi in mongoose. Summaries of select talks are provided below.
IV-a. Environmental nontuberculous mycobacteria hotspots of the Hawaiian Islands.
Among all U.S. states, Hawai’i has a history of leprosy and TB, but also shows the highest number of NTM lung disease cases. From her sampling of freshwater biofilms of residential housing in a pilot study, Dr. Honda found that M. chimaera and M. abscessus predominated, which mirrored NTM species responsible for lung disease in Hawai’i. Undergraduates and high school students participated in the collection of samples, totaling approximately 3,000 unique environmental samples, of which 27% were NTM positive. Larger cities were hotspots for NTM, with showerheads and sinks being the most common NTM positive locations in residential areas. Hematite in soil promoted the growth of environment-derived M. abscessus. Observations also indicate that human infections of M. abscessus were linked to NTM in kitchen sink faucets, while infections of M. chimaera were linked to NTM in showerheads. Animal reservoirs in Hawai’i include the feral pig populations of O’ahu. Honda et al., discovered a unique Hawai’i-specific environmental factor that may be associated with NTM is the Kīlauea volcano. Viable M. avium, M. abscessus, and M. chimaera were recovered from Kīlauea ash.
IV-b. Mycobacterium ulcerans lineages and Buruli ulcer: Q203 (Telacebec).
Until 2004, Buruli ulcer (BU) could only be treated by wide excision and skin grafting. Using the mouse footpad model followed by field trials, it was determined that rifampin and streptomycin (clarithromycin from 2017) administered daily for 8 weeks were more effective alternatives. A series of reports 36–39 have shown that Q203, now termed Telacebec, a bc1 oxidase inhibitor, could reduce the time to cure in immunocompetent and most immunocompromised mice to one week or even a single dose. M. ulcerans has a mutation in cydA resulting in the elimination of the bd oxidase inhibitor used by other mycobacteria as a backup electron transporter. Although the cydA mutation is present in the strains of M. ulcerans causing disease in Australia and West Africa (and, thus, >95% of reported cases), the mutation is absent in the Japanese subspecies, M. ulcerans shinshuense. Dr. Converse and his collaborators screened 9 strains of M. ulcerans for in vitro susceptibility to Telacebec. All were susceptible at a concentration of 0.078-0.156 ng/ml, except for a Japanese strain which was resistant to ≥50 ng/ml and a Mexican strain at 1.25-2.5 ng/ml. Further examination of the literature revealed that the Mexican and Japanese strains are part of the “ancestral” lineage 40 of M. ulcerans and that they are more closely related to M. marinum. The susceptible West African and Australian strains are in the “classical” lineage 40. Sequence analysis confirmed that the Mexican strain, like the published M. ulcerans shinshuense strain 41, has no stop codon at position 231 of the cydA gene, as found in classical lineage strains 42. It does have a distal codon deletion resulting in a frameshift at position 476, of yet unknown significance. There are some environmental differences between the lineages. Ancestral strains are associated with water whereas classical strains in Australia are associated with marsupials and likely mosquitos. Classical strains in Africa have not been found to be associated with mammals but have been associated with aquatic plants and snails as well as fish. The efficacy of BCG vaccination protection in mice appears to be mouse and M. ulcerans strain dependent 43. In humans in West Africa BCG appears to protect against osteomyelitis but not the more typical cutaneous disease 44. According to the BCG Atlas 45, both Mexico and Japan have historically administered BCG at birth and boosted at ~age 6 and, in Japan, ~age 13. Both countries use their strain of BCG while Mexico subsequently introduced the Danish strain. In West Africa, BCG vaccination is given only at birth. In Australia, neonatal vaccination was discontinued in 1985. From these data, one cannot infer whether boosted BCG vaccination may be protective or protective only against ancestral strains. A clinical trial of Telacebec in TB patients showed that it was safe 46. The drug is currently granted orphan drug status for BU, and a trial is clearly warranted to confirm its efficacy in BU patients and in leprosy where the drug has also been shown to be highly active in a mouse model 47.
IV-c. M. avium ss paratuberculosis and Johne’s Disease.
M. avium subspecies paratuberculosis (MAP) is the causative agent of paratuberculosis, a contagious, chronic, and eventually fatal enteric disease of domestic and non-domestic ruminants. MAP is highly resistant to heat, disinfectants, and environmental agents, remaining infective for a long time in the environment which make biosecurity a challenge when attempting to control this disease in domestic, zoo ruminants, and especially wild animals. The highest burden of MAP is in domestic ruminants, followed by zoo ruminant species, and then free ranging wild ruminants. The overall pathophysiology of MAP when it occurs in all three ruminant groups is essentially identical, so environmental factors would appear to have dominant roles in explaining the differences in the prevalence among the three groups.
MAP in zoological specimens has been reviewed 48 as well as in wildlife and farmed deer 49. Herd prevalence over a 10-year period in a group of comingled zoo ruminants nyala (Tragelaphus angasii), impala (Aepyceros melampus) and Thomson’s gazelle (Gazella thomsonii) demonstrate a clear difference between the low prevalence of MAP in the Thomson’s gazelle (~5%) compared to both the impala and nyala (~42%). When the Thomson’s gazelle herd were later fed a high intake of concentrate rations in a similar fashion to the nyala and impala, the prevalence of MAP then starts to approach these latter species (~25%). Hypocalcemia was apparent in the nyala and impala during the ten year study period but not a common feature of the Thomson gazelle until they were managed differently.
Feeding niches (grazers vs browsers) and nutrition, especially high concentrate low fiber feeding, leads to a higher prevalence of MAP as seen in these zoo ruminants. Hypocalcemia or increased calcium demand parallels this trend in MAP prevalence amongst these three comingled species at various times during the study period. Various aspects of diagnostic methodologies to stage the infection, including early life stage infection 50, and biosecurity are discussed in zoo animals, but the relationships between nutrition and MAP prevalence at zoos are rarely discussed, if ever. The observation is that the more intensively managed any ruminant becomes, especially in terms of feeding, the higher the apparent prevalence of MAP. This perspective may allow for exploring other control measures focused on feeding practices and more specifically on calcium metabolism. An increase in calcium demand is believed to be directly responsible for the changes in MAP prevalence seen in zoo ruminants and is a direct result of feeding practices. This hypocalcemia is known to alter immune response in cattle 51 and there is no reason to expect otherwise in other ruminants. In this regards, MAP infection in all ruminants can be more appropriately thought of as an anthropogenic condition.
IV-d. M. bovis infections in wild carnivores.
Although carnivore species have generally been thought to be more resistant to infections with pathogenic mycobacterial species, more recent research in both wild and domestic carnivores have demonstrated that both felids and canids can be infected with M. bovis and develop disease 52–54 (FIG 4). Due to their natural history, carnivores are often infected through ingestion of infected prey, although they also can be exposed through aerosol and cutaneous routes of infection 55, 56. Infected wild felids, including lions, leopards, and cheetahs, often have evidence of generalized disease, with cavitary pulmonary lesions, lymph node granulomas, which lack mineralization and appear proliferative, as well as osteolytic lesions in bones and granulomas in abdominal organs 56, 57. M. bovis infection in critically endangered African wild dogs (Lycaon pictus) appears to be a rapidly progressive disease, which has resulted in mortality of even young animals 54.
FIGURE 4. M. bovis infections in wild carnivores.
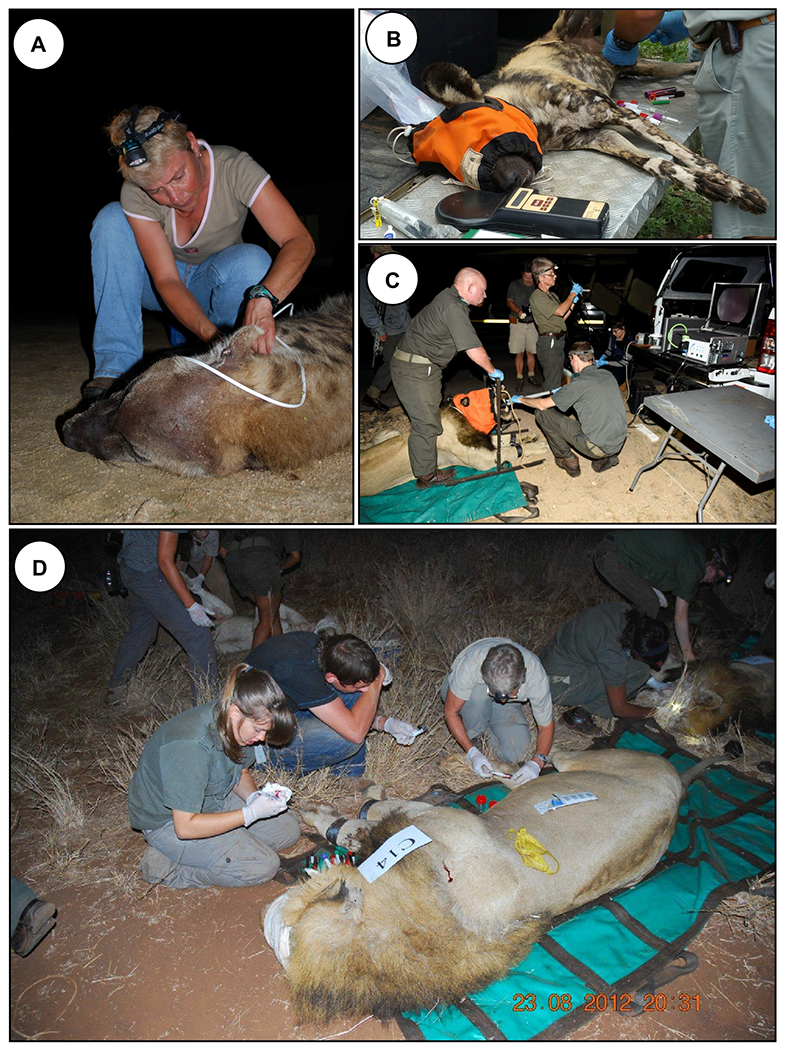
To survey the prevalence of M. bovis infection and identify their associated cell-mediated immunological biomarkers in wild carnivores or Kruger National Park, South Africa, teams of veterinarians and researchers anesthetize and collect blood from and/or perform endoscopies on (A) spotted hyenas, (B) African wild dogs, and (C-D) lions. Samples are then brought back to laboratory for microbiological and immunological assessments. Images courtesy of Michele Miller.
Since M. bovis has been isolated from ante-mortem respiratory samples of infected lions and African wild dogs, the assumption that these are dead-end hosts has been challenged 58, 59. Limited studies have shown that in vitro and in vivo cell-mediated immune responses can be used to diagnose infection in wild carnivores. Further comparative immunological studies of spotted hyenas’ (Crocuta crocuta) response to M. bovis infection may provide insight into natural resistance of some species to development of disease 60.
V. MYCOBACTERIAL DISEASE COMORBIDITIES AND CONFOUNDERS
Mycobacterial disease often occurs against the backdrop of one or more comorbidities. These comorbidities may be genetic, dietary or environmental in origin, and can drive mycobacterial disease transmission or progression, as well as impact mycobacterial disease surveillance, treatment and outcomes. Presenting their research on this subject were Dr. Luanne Hall-Stoodley (OSU), Dr. Jyothi Nagajyothi (Hackensack Meridian Health), Dr. Kevin Fennelly (NIH/NHLBI), Dr. Brendan Podell (Colorado State University), and Dr. Murugesan Rajaram (OSU), who spoke on the subjects of NTM and cystic fibrosis (CF), adipocyte dysfunction and TB, NTM and chronic obstructive pulmonary disease (COPD), TB and metabolic disorders, and M. avium and cardiac dysfunction. Summaries of select talks are provided below.
V-a. Mycobacterium abscessus and cystic fibrosis.
NTM have become the 3rd leading cause of lung infection in people with cystic fibrosis (pwCF) 61, 62. Macrophages from pwCF show several deficiencies in macrophage function in response to bacteria 63 and previous work showed that M. abscessus (MAB) smooth and rough morphotypes each can survive in human macrophages 64. Dr. Hall-Stoodley found that MAB also survives in primary human monocyte-derived macrophages (MDM) and that while CF macrophages can harbor both morphotypes, CF macrophages fail to significantly restrict intracellular growth of the MAB rough morphotype compared to smooth. Failure to restrict the more virulent rough MAB was recapitulated with infection with rough MAB and knock-down of cystic fibrosis transmembrane conductance regulator (cftr) 65. Treatment of infected non-CF macrophages with a CFTR inhibitor resulted in a significantly reduced respiratory burst and infection with rough MAB resulted in a more pronounced reduction. Finally, infected CF macrophages showed reduced phosphorylation of NOX2 p47phox. These findings suggest that CFTR dysfunction may contribute to the susceptibility of pwCF to persistent infections with virulent rough MAB.
V-b. Adipocyte dysfunction induces insulin resistance in Mtb infection.
The risk of developing active TB disease from latent TB infection (LTBI) is three times higher in individuals with type 2 diabetes mellitus (T2DM). The association between TB and T2DM is becoming more obvious as T2DM is rapidly growing in settings where TB is endemic. T2DM is a chronic metabolic disorder characterized by elevated blood glucose, insulin resistance, and relative insulin deficiency. Insulin resistance and stress-induced hyperglycemia have been shown to be increased by TB and to return to normal upon treatment. It has been shown that Mtb persists in adipose tissue within adipocytes and regulates pulmonary pathology, inflammation, and load in a murine model of TB 66. Since adipose tissue pathology is involved in T2DM pathogenesis we examined whether metabolic alterations in adipose tissue via a high-fat diet alters pulmonary pathology and insulin signaling in Mtb-infected mice. We also investigated whether these changes alter between juvenile and adult mice since the levels of adipose tissue are greater in adult mice. Here, we report the impact of Mtb infection on the development of insulin resistance in mice fed on a regular diet (RD) versus a high-fat diet (HFD) and, conversely, the effect of hyperglycemia on pulmonary pathogenesis in juvenile and adult mouse models. Overall, our study demonstrated that Mtb persists in adipose tissue and that Mtb infection induces irregular adipocyte lipolysis and loss of fat cells via different pathways in RD- and HFD-fed mice. We also showed that Mtb-infected adult mice that were fed an RD developed insulin resistance similar to infected adult mice that were overweight due to an HFD diet. Our findings may underpin the clinical observation that non-obese/non-high-BMI individuals with LTBI are more likely to develop hyperglycemia and diabetes compared to those without Mtb infection 67.
V-c. TB and metabolic comorbidities.
Prior to the clinical deployment of insulin therapy, diabetes was recognized as a major contributor to TB-associated mortality 68. Now the re-emergence of diabetes as a risk factor due to convergence in TB-endemic regions has brought to the forefront metabolic risk factors with high global prevalence, contributing up to 40% of the collective population attributable risk 69. Among these, the collective risk attributed to states of malnutrition should be reconsidered in the context of specific micronutrients, as exemplified by the strong individual risk association for TB disease progression when vitamin A deficiency predates Mtb exposure 70, 71. Chronic uncontrolled diabetes in both mice and guinea pigs leads to more severe TB disease outcomes that result from a hallmark of delayed adaptive immune onset, precluding timely control of bacterial growth in tissue 72–74. The destructive pathology in diabetes-TB comorbidity is distinct from the influence of vitamin A deficiency on TB progression 72. In the latter, a lack of the canonical guinea pig pathology features, including granuloma necrosis, are notably absent in the context of vitamin A deficiency. This may be attributable to compensatory increases in CD8-driven cytotoxic immunity and reduced neutrophil involvement 70. Of note, both diabetic hyperglycemia and vitamin A status exist in a bidirectional relationship with TB, where TB causes diabetes-like hyperglycemia and also reduces serum vitamin A even with sufficient dietary intake 70, 75. The bidirectional nature of these TB comorbidities has potential to create diagnostic challenges but also may illuminate novel approaches for host-directed therapy 76.
V-d. M. avium infection and cardiac dysfunction.
The M. avium complex (MAC) consists of M. avium and M. intracellulare and enters the host primarily through the gastrointestinal track and pulmonary route. MAC is a common cause of mycobacterial infection in immune compromised individuals including, the elderly and those with COPD, HIV/AIDS or CF. Mycobacterium can infect every organ in the body including the heart causing carditis. Myocardial TB is difficult to diagnose and treat and can cause heart failure and ventricular tachycardia which can ultimately lead to sudden cardiac arrest. Very little is known regarding cardiac dysfunction during mycobacterial infection in the elderly. In this study we examined cardiac electrical activity, cardiac inflammation, and fibrosis in young and old mice after M. avium infection. However, electrocardiogram of old mice shows abnormalities that include sinus node pause and first-degree heart block. Furthermore, we identified that the number of MHCIIlow cardiac resident macrophages (CRMs) are decreased in old mice upon infection, in contrast we found an increased number of inflammatory MHCIIhigh CRMs which correlated with an increased cardiac inflammation and fibrosis. Thus, we hypothesize that mycobacterial infection leads to increased cardiac inflammation, fibrosis and necrosis which lead to significant electrical disturbances in old mice. Our data revealed that enhancing the expression of efferocytic receptor MerTK in CRMs reverses the endotoxin mediated cardiac inflammation and electrical dysfunction in old mice, which suggests that the molecules that increases the expression of MerTK could be a potential therapeutic agent for cardiovascular disease in elderly people.
V-e. Nontuberculous mycobacteria and COPD.
Chronic obstructive pulmonary disease (COPD) is a major cause of morbidity and mortality in the United States, affecting more than 15 million adults. COPD is one of the most common conditions associated with pulmonary NTM disease, most likely due to impaired mucociliary clearance of inhaled or aspirated bacilli. In a study of NTM patients in the US Veterans Administration, 68% had COPD, with the highest rates in the Gulf Coast states 77. Because NTM are environmental organisms, diagnosis from sputum requires finding the same species in at least two different specimens. Cavitary disease is common, and M. abscessus has been found to exist in biofilm within a lung cavity that was surgically resected from a COPD patient 78. Treatment is difficult, often requiring multiple drugs for many months to years.
VI. MODELS FOR ASSESSING MYCOBACTERIAL DISEASE AND COINFECTIONS
Animal models of mycobacterial disease have contributed greatly to our current understanding of mycobacterial disease pathogenesis and immunity, as well as how coinfections with other common pathogens influence mycobacterial disease. To address this subject and provide updates on their research in this space, panel members Dr. Fred Quinn (University of Georgia), Dr. Karen Dobos (Colorado State University), Dr. Janice Endsley (University of Texas Medical Branch at Galveston), Dr. Selvakumar Subbian (Rutgers University), Dr. Vidya Vijayan (UNC Chapel Hill), and Dr. Deanna Hagge (The Leprosy Mission International) presented, respectively, on using ferrets to model the Mtb carriage state 79, the current state of biomarker utility in the guinea pig infection model 80, the identification of novel macrophage signaling pathways in humanized mice with HIV/Mtb coinfection 81, 82, the rabbit model of latency and reactivation to evaluate the effect of host-directed therapy 83, 84, epigenetic modifications in BCG vaccinated infants 85, and the complicity of soil-transmitted helminths in leprosy treatment complications 86.
VI-a. Modeling the Mtb carriage state.
Major respiratory bacterial pathogens such as Streptococcus pneumoniae and Haemophilus influenzae utilize an upper respiratory tract (URT) carriage state in which the bacteria attach to the local epithelia, survive and slowly replicate, and transmit person-to-person, but do not damage the host cells, cause disease in this location or induce more than a very mild local inflammation; these commensal bacteria ultimately clear or they can disseminate to sterile areas of the body and cause disease. Importantly, vaccines have been shown to clear URT colonizing bacteria and thus prevent subsequent disseminated disease states or transmission to naïve contacts. Data from TB studies not originally designed to assess carriage have inadvertently demonstrated that Mtb bacilli may also possesses a URT carriage state in humans, and the Quinn lab demonstrated carriage in natural transmission studies in ferrets; understanding this state is critical to ultimately controlling disease development, transmission and vaccine efficacy.
VI-b. Current state of biomarker utility in the guinea pig infection model.
The guinea pig model of TB has been used for decades as a small animal model for testing vaccines. Indeed, it is an attractive model, as BCG vaccination demonstrates significant protection from development of severe TB, and because the lung pathology of infected guinea pigs resembles human disease with primary and secondary granulomas along with similar histopathology. However, this model lags in the use of other biomarkers and the benefits of their use; notably 1) measuring secreted and cellular cytokines and other markers of disease progression or protection, limiting its use to define correlates of protection beyond reduction in CFU and histopathological analyses of organs harvested from euthanized animals, and 2) performing longitudinal studies. In this presentation, current biomarker analysis by the Dobos lab using the guinea pig model of TB and some prospects were presented for discussion.
VI-c. Humanized mice and HIV/Mtb coinfection.
Mycobacterial infections have a multifactorial synergy with Human Immunodeficiency virus (HIV) that is incompletely understood. We developed humanized mouse models of HIV and Mtb co-infection to facilitate discovery of disease mechanisms and develop interventions 87–89. Differential transcriptomics analysis of lung from humanized mice infected with Mtb or Mtb and HIV identified disturbances in immune signaling of macrophages, including the C-type lectin receptor (CLR) pathways. Subsequent investigations identified a novel role for the CLR Macrophage Galactose Lectin (MGL) in innate immunity to Mtb 82that is dysregulated in the setting of HIV co-infection. Using a paucibacillary model, the Endsley lab further identified a potential role for T helper 17 (Th17) and T helper type 22 (Th22) pathways as a mechanism for HIV-mediated TB relapse. On-going research is focused on determining whether interleukin (IL-17) pathways play a role in maintaining immunological control of Mtb after treatment, as well as the role of MGL in foamy macrophage development and anti-mycobacterial activity. Additional research activities include investigation of drugs that can be used to treat both TB and HIV in a coinfection setting 90.
VI-d. Rabbit model of latency and reactivation to evaluate the effect of host-directed therapy.
A fourth of the world population is estimated to be latently infected with Mtb without the symptoms of active TB, which occurs in about 5-10% of infected individuals. A recent clinical trial has indicated that adjunctive treatment of Everolimus (EVR) with standard antibiotics-based therapy to patients with TB improved their clinical outcomes. However, the effect of such host-directed therapy on the reactivation of LTBI remains unknown. Here, results from the Subbian lab were presented regarding the effect of adjunct EVR treatment, in combination with two first-line drugs, isoniazid (INH) and rifampicin (RIF), on latency and reactivation of TB. The Subbian lab used a well-established rabbit model of pulmonary Mtb infection for these studies (FIG 5). Results of this study include the pharmacokinetics and pharmacodynamics determination of EVR, INH and RIF in the plasma, as well as the longitudinal analyses of bacterial load and histopathology of the lungs. The findings and their implications on LTBI/reactivation were discussed.
FIGURE 5. The outcome of pulmonary infection with Mtb in rabbits.
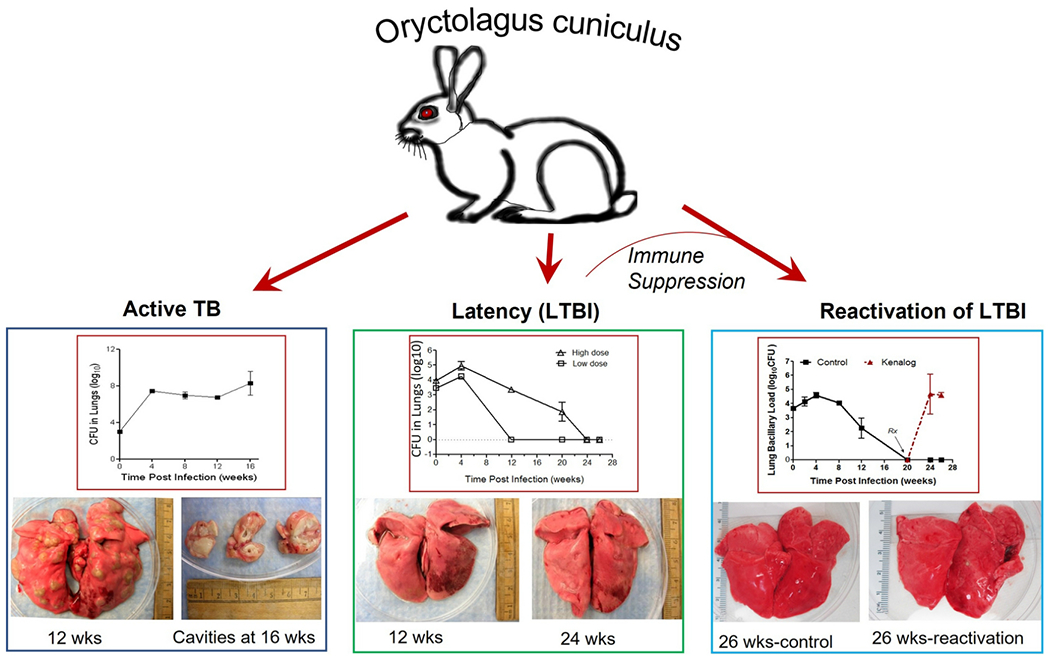
Depending on the nature of infecting Mtb strain and the infectious inoculum used, rabbits (Oryctolagus cuniculus) can develop the progressive cavitary disease (Active TB; left panel), marked with an elevated bacillary load at 4 weeks that persists until 16 weeks post-infection. These rabbits develop heterogeneous lung granulomas, including necrotic, caseating, and cavitary lesions. The latent Mtb infection (LTBI; mid-panel) model in rabbits is characterized by a transient bacillary growth until 4 weeks that gradually reduces to a complete absence of live bacteria over time (12-24 weeks). These rabbits rarely have any subpleural lesions. Immunosuppression (Kenalog) treatment of rabbits with LTBI leads to the reactivation of active disease (right panel) with elevated bacillary growth in the lungs. The lightweight line is to indicate that immune suppression of rabbits with LTBI results in reactivation of disease. Images courtesy of Selvakumar Subbian.
VI-e. Epigenetic modifications in BCG vaccinated infants.
BCG, a live attenuated vaccine, was introduced a century ago to prevent TB and is still administered to >80% of newborns worldwide. BCG is highly effective in preventing severe complications associated with TB disease in infants, and overall childhood mortality dropped significantly since the introduction of the BCG vaccine, especially in resource-poor countries. Studies in adults provided insights into the underlying mechanisms of BCG effectiveness and studies revealed that BCG induces epigenetic changes in monocyte populations which results in an improved functional capacity that persists for several months. Importantly, BCG-induced enhanced innate effector function is not restricted to TB antigens but extends to unrelated pathogens, a phenomenon now referred to as “trained immunity.” The improved innate immune function also promoted a shift towards T helper (Th) 1 and Th17 T cell responses to TB, but also to BCG vaccine-unrelated antigens, a phenomenon known as heterologous immunity. Although highly likely, to our knowledge, BCG-induced epigenetic changes found in adult monocytes have not been confirmed in infants that represent the main target population for BCG. BCG-vaccinated infants also have potent Th1 interferon gamma (IFNγ) responses. Therefore, the Vijayan lab tested monocytes and CD4 T cells of 20 infants prior to and 6-15 weeks post-BCG vaccination for epigenetic changes by performing transposase-accessible chromatin using sequencing (ATAC seq) and for transcriptome changes by RNA sequencing. These data were presented as were their plans for ongoing analyses.
VI-f. Complicit Indicators: Leprosy and Soil-Transmitted Helminths.
Chronic soil-transmitted helminths (STH) infections can induce long-term immune modulation and suppress host cellular immunity; however, deworming can disturb or eliminate STH-induced immune suppression. Results from the Hagge lab were presented demonstrating that more than 94% of annual new leprosy cases are diagnosed in populations co-endemic for STH; and significant associations have been established with increased M. leprae burdens (multibacillary or lepromatous leprosy) and inversely with presence of immunological complications (leprosy reactions) at leprosy diagnosis. Also presented was a second large study by the Hagge lab in Nepal incorporating aspects of deworming, Water and Sanitation and Hygiene (WASH) training, multiplex qPCR detection of endemic STH species, and longitudinal follow-up for up to 2 years. From 2003-2020, there was a first Mass Drug Administration (MDA) campaign in Nepal which gradually worked across districts with repeated annual rounds of treatment with albendazole. Therefore, retrospective mining of medical charts over decades from a referral leprosy hospital are also being investigated for trends.
VII. LEVERAGING THE LESSONS OF MYCOBACTERIA TO IMPROVE HEALTH
The overall theme of this session was how we might apply our existing knowledge of mycobacteria and the host response to derive novel medical and engineering interventions. Panel members included Dr. Mark Endsley (University of Texas Medical Branch) and Dr. Andrew Simonson (University of Pittsburgh), who respectively spoke of their work relating to inhalable aerogels for rapid clearance of pulmonary TB 91, the role of lymphocyte subsets in preventing TB following intravenous vaccination with BCG 92, and TB chemotherapy in an HIV setting 87, 90. Additional panel members included Dr. Don Hayes Jr (Cincinnati Children’s Hospital Medical Center), Dr. Natalie Hull (The Ohio State University) and Dr. Christopher Lowry (University of Colorado), who presented on the subjects of mycobacteria and lung transplantation (Hayes), novel technologies for disinfection of NTM laden water (Hull), and the modulation of the psychological stress response by M. vaccae 93, 94 (Lowry). Summaries of select talks are provided below.
VII-a. Mycobacteria and Lung Transplantation.
Patients with structural lung disease are at risk for acquiring mycobacteria in their lower airways, so acquisition of these pathogens can be challenging for those seeking lung transplant (LTx) due to the limited number of therapies available and the increasing resistance to those therapies. The recent consensus document for the selection of LTx candidates published by the International Society for Heart and Lung Transplantation reported that active TB infection is an absolute contraindication for LTx, whereas M. abscessus infection falls under the category of high or substantially increased risk 95. Therefore, patients with pulmonary NTM undergo LTx at transplant centers comfortable in managing the pathogen afterwards. At present, there are no standardized guidelines for diagnosis and management of pulmonary NTM during the pre-LTx evaluation and management period. Those patients who are identified as potential LTx candidates with pulmonary NTM should be adequately treated with multidrug regimens. An older publication recommended consideration of listing patients for LTx if three sputum samples are negative for AFB stain with negative mycobacterial cultures for more than one year 96. However, it is not uncommon for potential LTx candidates to never clear pulmonary a NTM species, so some transplant centers will consider LTx in these patients if three consecutive monthly sputum samples remain negative for AFB. Due to variable post-LTx outcomes in patients with pulmonary NTM, especially M. abscessus, transplant centers have been more cautious in considering patients for LTx with these particular pathogens. A single-center study found these inferior post-LTx outcomes were associated with M. abscessus subspecies abscessus, especially ST-1 and ST-26 strains, while other M. abscessus strains had acceptable outcomes 97.
A systematic review and meta-analysis included a total of 10 studies and found that pulmonary NTM disease (as defined by international guidelines) and not isolation (as defined by growth of NTM in culture) was associated with worse outcomes 98. This meta-analysis found risk factors associated with the development of NTM disease, including cystic fibrosis and pre-transplant NTM isolation 98. Therefore, strategies to optimize prevention and treatment of NTM disease in LTx recipients are needed. Phage therapy targeting pulmonary NTM was recently attempted under compassionate use which was found to be challenging due to the limited repertoire of therapeutically useful phages, but this group found favorable clinical outcomes in patients with their findings supporting their use as an adjunctive therapy for some mycobacterial infections 99. Our personal anecdotal experience using an intravenous mycobacteriophage in trying to clear the sputum positive AFB stain in a potential LTx candidate with CF colonized with M. abscessus subspecies abscessus was only partially successful (FIG 6). Although the sputum was cleared, the patient eventually developed antibodies that neutralized the Mycobacteriophage. Therefore, phage therapy in a chronic setting may be limited by the immune response and require modifications to the phage itself. Future therapies may in the horizon with a recent study identifying deficiencies in a number of lung resident immune cells 100, so this work hints at the possibility of targeting a lack of those immune cells, such as a cell therapy, as a means to treat pulmonary NTM.
FIGURE 6. A case of mycobacteriophage-resistant M. abscessus infection.
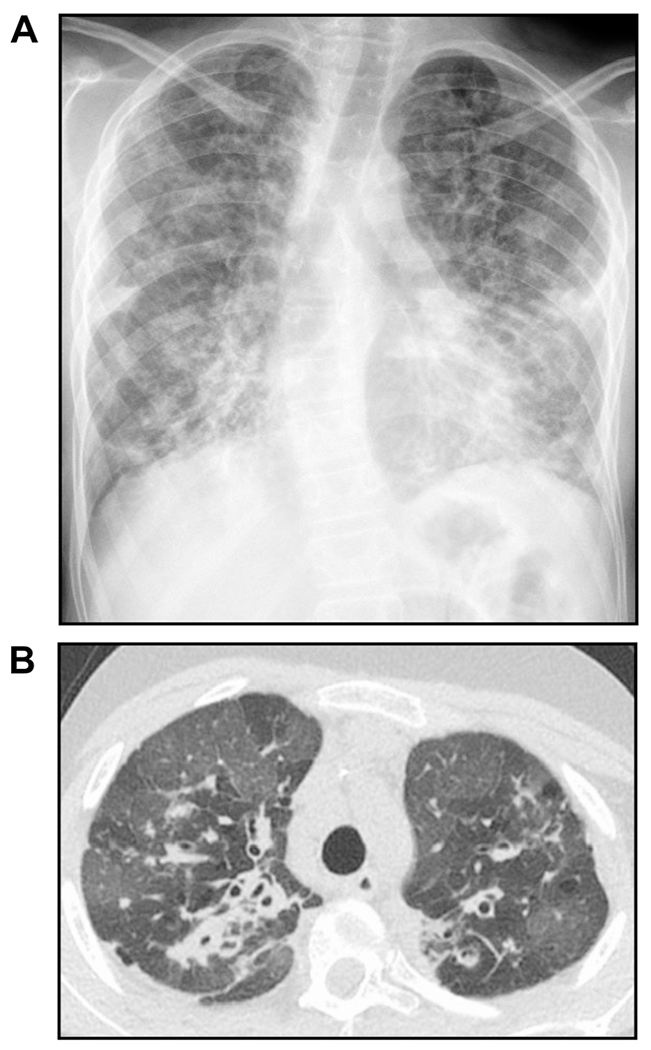
(A) Plain chest radiograph and (B) computed tomography of the chest showing advanced bronchiectasis in an adolescent with cystic fibrosis and CFTR genetic mutations not amenable to CFTR modulator. The patient presents for lung transplant evaluation, but that was delayed due to sputum being both AFB stain positive and culture positive for M. abscessus. Standard and investigational therapies were unsuccessful in clearing the positive AFB sputum stain to allow consideration for transplant at that time, so intravenous Mycobacteriophage therapy was instituted that eventually cleared the positive AFB sputum stain. However, after one-year of phage therapy, the patient developed neutralizing antibodies against the phage leading to the return of the AFB sputum stain positivity. Images courtesy of Don Hayes Jr.
VII-b. Battling opportunistic NTM infections with novel ultraviolet light emitting diodes.
Pulmonary infection with NTM is a growing problem, the incidence of which varies geographically 101–105. The route by which NTM enter the lung, however, is not always clear and varies on a case-by-case basis, the possibilities including silent aspiration (since NTM infections have been associated with gastroesophageal reflux disease) 106, carryover from contaminated bronchoscopes 107, and inhalation exposure to environmental aerosols 108. Potential NTM-containing aerosols include those originating from showers, hot tubs or therapy pools, or aspiration of drinking water 109. Mycobacteria are prevalent in municipal waters (including Ohio 110, the state which hosted MHM9), enriched by water treatment and distribution, prolific in building plumbing, and enriched in showerhead biofilms and shower hoses 35, 111. Since NTM are resistant to antibiotic therapy and proliferate in plumbing systems under the responsibility of building owners, a point of use (POU) engineering solution to disinfect residential water supplies would be the quickest (and likely most economical) solution to mitigate the risk of NTM inhalation in these environments 112.
Ultraviolet (UV) light emitting diodes (LEDs) pose a novel and sustainable potential solution for water disinfection at a POU due to their small size and low power requirements. UV disinfection requires no chemical additions, causes no taste/odor issues, has no harmful effect from overdosing, does not apply selective pressure for chemical resistance, and has little to no effect on disinfection byproduct formation 113. Dr. Hull presented the results of a year-long demonstration test comparing the world’s first commercial UV LED water disinfection reactor with an existing chlorination system at a small water treatment plant 114. The study achieved success during lab tests under controlled conditions and in the field under challenging conditions without any maintenance, the reactor demonstrated viral and bacterial disinfection efficacy and resilience equivalent to the chlorination system, providing proof of concept for application of UVC LEDs for municipal water treatment. The success of this study raises the likelihood that this technology could be applied at the POU, and is being adapted by the Hull laboratory to electricity-free environments (e.g. a shower stall or water tap) by converting the hydropower inherent to residential water systems into the energy which powers the UV LED, to protect individuals susceptible to aerosol-borne opportunistic pathogens such as NTM.
To incorporate UV LEDs in a POU solution to mitigate the risk of NTM inhalation, the efficacy of UV LEDs for disinfecting NTM and limiting NTM infectivity must first be determined. NTM are susceptible to the radiant energy produced by UV light 115; however, previous tests of UV-based solutions to sterilizing NTM-containing water were performed with nonpathogenic NTM isolates and hazardous mercury UV lamps which are not practical in a residential setting, both from an environmental perspective (mercury from breaking fragile quartz lamp bulbs is toxic) and energy efficiency perspective. Since the UV spectrum of mercury lamps is limited by the physical properties of this element (254nm for low pressure lamps), it is also possible that other UV wavelengths emitted by LEDs may be more effective at sterilizing NTM-containing water. For example, bacteria are more sensitive to 260 nm due to greater absorbance of nucleic acids versus 254 nm 116, 117. For UV treatments, disinfection differs from preventing infection because UV damages nucleic acids (inhibiting replication) and proteins (potentially altering host response) 118, but does not immediately render cells dead. Because previous studies relied on CFU as their readout (i.e. a measure of NTM capacity to grow on nutrient rich agar), it is unclear the extent that different UV wavelengths or intensities are sufficient to limit NTM infectivity (i.e. a measure of NTM capacity to cause lung disease in an animal or tissue culture model). Hull aims to determine the efficacy of UV LEDs for disinfecting pathogenic NTM isolates in water and limiting their infectivity, enabling further research and design of a sustainable engineering solution to combat increasing incidences of NTM disease.
VII-c. Mycobacteria as “Old Friends”: Mycobacterium vaccae NCTC 11659, a soil-derived bacterium with anti-inflammatory and stress resilience properties.
The “Old Friends” hypothesis proposes that persons living in modern, industrialized societies are at increased risk of developing inflammatory conditions 119, as well as stress-related psychiatric disorders in which chronic, low-grade inflammation is considered a risk factor 120. Increased risk of inflammatory conditions in modern, industrialized societies is thought to be due to reduced exposure to diverse microbial environments, and subsequent failure of immunoregulation. Immunoregulation, as defined by a balanced expansion of effector T-cell populations and regulatory T cells (Treg), is driven by microbial signals, principally by microorganisms with which mammals, including humans, coevolved (FIG 7). As defined by the “Old Friends” hypothesis, microorganisms with immunoregulatory properties include: 1) the commensal microbiota, which have been altered by the Western lifestyle, including antibiotics and a diet that is commonly low in microbiota-accessible carbohydrates 121, 122; 2) pathogens associated with the “old infections” that were present throughout life in evolving human hunter-gatherer populations 123, 124; and 3) organisms from the natural environment with which ancestral humans were in daily contact with (and, consequently, had to be tolerated by the immune system) 125. Immunoregulation is thought to be compromised in modern high-income settings due to reduced contact with these three categories of organisms 125–127. Evidence suggests that some strains of non-pathogenic mycobacteria may be considered “Old Friends”, with anti-inflammatory, immunoregulatory, and stress resilience properties. These include the soil-derived mycobacterium, M. vaccae NCTC 11659, and the type strain, M. vaccae ATCC 15483 128, 129. Several lines of evidence suggest that many strains of mycobacteria, if not “Old Friends”, may be tolerated by the immune system. For example, recent studies have shown that mycobacteria are the most abundant bacterial taxa in municipal water supply systems in both the U.S. and Europe 35, while a study of healthy humans identified fifty abundant mycobacteria in the oral cavity (buccal mucosa and dental plaque) and upper respiratory tract (nostrils and oropharynx) 130. It remains to be determined if other strains of mycobacteria also have anti-inflammatory, immunoregulatory, and stress resilience properties.
FIGURE 7. Hypothetical model illustrating mechanisms through which “Old Friends”, such as M. vaccae NCTC 11659, impact systemic inflammation and immunoregulation.
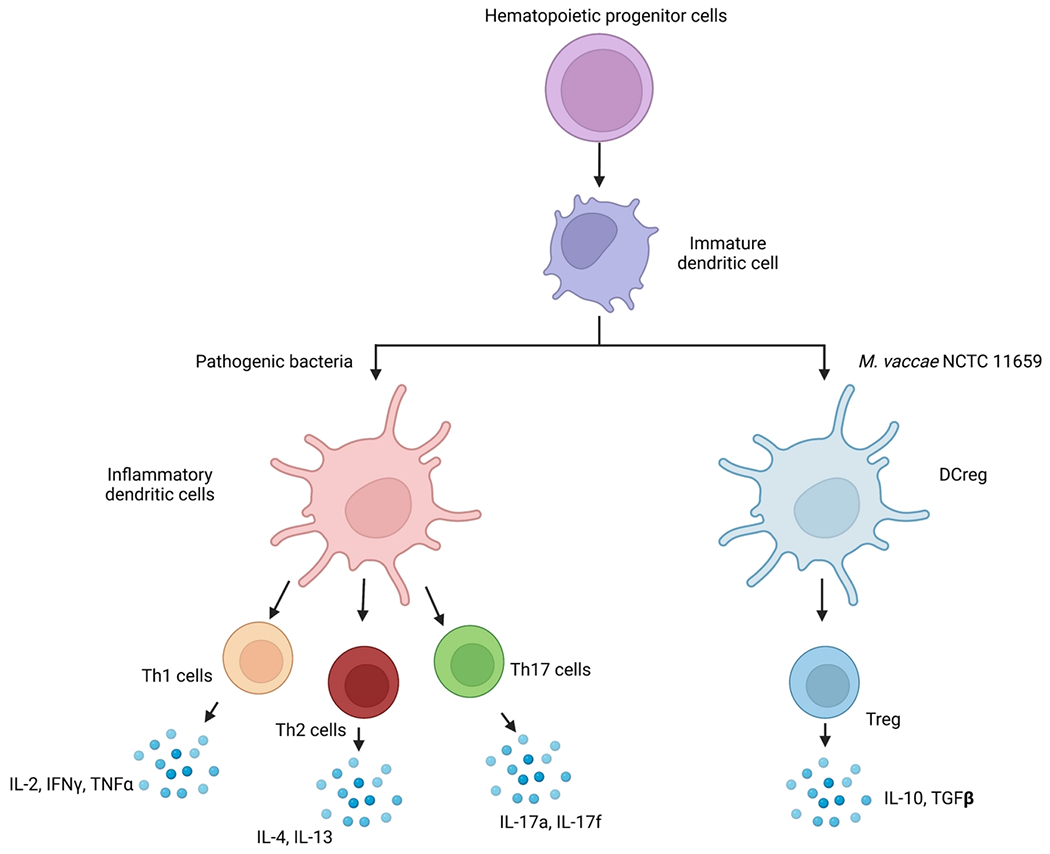
Exposure of immature dendritic cells (DC) to M. vaccae NCTC 11659 induces differentiation of regulatory DCs (DCreg) that bias T-cell differentiation away from T helper type 1 (Th1), Th2, and Th17 cells towards induction of regulatory T cells (Treg) that secrete anti-inflammatory cytokines including interleukin 10 (IL-10) and transforming growth factor beta (TGFβ). Abbreviations: DCreg, regulatory dendritic cells; IFNγ, interferon gamma; IL-2, interleukin 2; IL-4, interleukin 4; IL-10, interleukin 10; IL-13, interleukin 13; IL-17a, interleukin 17a; IL-17f, interleukin 17f; NCTC, National Collection of Type Cultures, Th1, T helper type 1; Th2, T helper type 2; Th17, T helper type 17; TNFα, tumor necrosis factor alpha; Treg, regulatory T cells. Figure made with Biorender. Image courtesy of Caelan Wright.
VII-d. Sterilizing immunity following intravenous vaccination with BCG.
In the face of limited success by vaccine candidates to achieve target metrics in lengthy clinical trials, various approaches have been investigated to augment the efficacy of the BCG vaccine. Recent work has demonstrated the unprecedented level of sterilizing immunity conferred by intravenous, rather than intradermal, administration of BCG in a non-human primate challenge model 92. Robust T cell responses observed in the lung and airway indicate that long lasting cellular immunity is critical in preventing severe, progressive disease. Notably, significant increases in mycobacterial-responsive cells were seen in both CD8 and CD4 populations. It is therefore critical to investigate the role of T cell subsets in conferring this impressive immunity in order to guide future vaccine research and understand how host control of TB is achieved.
VIII. LEPROSY AND ITS ASSOCIATED DISPARITIES
At MHM9, the presentations and discussions on leprosy were led by Dr. John Spencer (Colorado State University), Dr. Carlos Franco-Paredes (Hospital Infantil de México Federico Gómez, México), Dr. Kathryn Dupnik (Weill Cornell Medicine), Dr. Ramanuj Lahiri (National Hansen’s Disease Programs), and Dr. Maria Pena (National Hansen’s Disease Programs). Presentations were on the subjects of “Contrasting conditions in Norway in the 19th-20th century and currently in Brazil”, “Clinical experience in treating patients with leprosy in the U.S., Mexico, Colombia, and Cuba”, “Blood transcriptome in leprosy reversal reaction”, “Leprosy in The United States of America”, and “The Armadillo Model For leprosy neuropathy.” Particularly inspiring to the educators and learners in the audience was the account of M. leprae discovery in Norway being tied to mentorship, the spirit of which continues today as the next generation of leprosy physicians and researchers (FIG 8). This history is recounted below, along with a summary of the current situation in Brazil and US.
FIGURE 8. Training the next generation of healthcare workers in Brazil and Panama to recognize signs and symptoms of leprosy.

Scenes from an expedition in Brazil to a fishing village where local healthcare workers were trained to recognize the signs of leprosy. (A) Fishing villages are often accessible only by boat and comprise (B) poor homes in close proximity to frequently flooded river habitats. For this particular trip, (C) a team of leprosy experts and specialists are led by community health agents to the homes of suspected cases. (D) Suspected cases are either examined at their home or (E) in a clinical setting. (F-G) A point-of-care tool being used to improve leprosy diagnosis (Semmes-Weinstein monofilament), seen here being applied to suspected patches of M. leprae infected skin on the (F) cheek and (G) foot. As originally reported in 2021, a Semmes-Weinstein monofilament kit comprises six different monofilaments from the thinnest one, light green (0.07 gm force, like the feeling of a mosquito landing on your skin) to the thickest, pink (>300 gm, the thickness of a pencil lead). The graded force of the six colored monofilaments are used to progressively determine loss of sensation in skin lesions or on the hands or feet by touching the monofilament tip to the skin surface and asking the person if they feel it. If they don’t feel the thickest pink one, then there is total loss of sensation in the skin lesion. Photos courtesy of John Spencer and Carlos Franco-Paredes.
VIII-a. Leprosy in Norway in the 19th - 20th century.
In the mid-19th century, as the prevalence of leprosy began to decline in Western Europe and the UK, the disease spread northward into Scandinavian countries, particularly Norway, Finland and Iceland. In Norway, respected dermatologists Carl Boeck (1808-1875) and Daniel Danielssen (1815-1894) published a book, “Om Spedalske” (About Leprosy) with illustrations detailing the main features of skin lesions and deformities found in lepromatous patients. Included were descriptions of damage to nerves causing lagophthalmos (inability to close the eyelids completely due to injury to the seventh facial nerve), atrophy of muscles of the fingers with contractures and progressive bone loss and destruction of the bones and cartilage of the anterior nasal spine and alveolar process of the maxilla leading to structural loss of the nose and frontal incisors. Danielssen later mentored a young physician, Gerhard Henrik Armauer Hansen (1841-1912) who was able to see rod shaped bodies (bacteria) with a microscope from skin scrapings of lesional material from leprosy patients. Hansen first submitted a preliminary report on his findings in 1873 and soon after published papers indicating the possible discovery of the etiological agent of leprosy 131, 132. Despite not being able to cultivate the bacteria on any artificial media, he was attributed with the discovery of Mycobacterium leprae, the first bacterial human pathogen associated with this ancient disease.
Prior to the 1820s, leprosy was not considered a serious health issue in Norway but censuses of leprosy patients in 1836, 1845 and 1852 reported increasing numbers of those afflicted leading to the initiation of control measures with 1,782 new cases that final year. The National Leprosy Registry of Norway was established in 1856 133. Initial responses of health officials in Norway in the mid-1800s were to isolate all individuals suspected of having leprosy regardless of the form of disease and to congregate them in many isolated farmsteads which likely resulted in exacerbating high rates of transmission. Eventually, three new hospitals were built from 1854-1861 with a total number of 930 beds. With the establishment by Hansen that the disease was spread by an infectious agent, health authorities began to believe that transmission could be reduced by isolating and congregating contagious individuals in these hospitals. The admission of individuals with leprosy was voluntary until 1875, after which time patients either had to be committed to a hospital or quarantined at home. Peak new case detection rates (NCDR) were 2-3 per 10,000 population for all age groups above 15 years old from 1856 to 1875, rates currently considered highly endemic 134. New cases in children under 15 years old also reached a peak during this time at around 8% indicating continued unchecked spread of multiple foci in the general population. After 1886, age related demographics shifted with fewer cases detected in children (<0.07 per 10,000 population between 1901 to 1920) and increasing in individuals over 60 years of age representing the highest percentage of the total of new cases detected at 30% 135. Factors contributing to the decrease of leprosy in Norway were a steady increase in per capita income, improved nutritional status and lower population density in dwellings all leading to better socio-economic conditions up to the beginning of the 20th century. After 1920, only 27 new cases of autochthonous leprosy were recorded in Norwegians 134 indicating elimination in the modern era, a remarkable achievement considering it was decades before successful drug therapy for leprosy was discovered.
VIII-b. Leprosy in Brazil in the 21st century.
Brazil remains the only country in the world that still has not met the World Health Organization’s goal of less than one new case per 10,000 population, with rates in 2019 around 1.3 new cases per 10,000. Prior to the pandemic, Brazil averaged 27,000 new cases per year from 2015-2019. Of these between 7.4% and 5.5% were in children under 15 years old 136, an important epidemiological indicator as this correlates with recent disease transmission and multiple active foci of spread within the community 137. There remain disparities in NCDR in different states in Brazil. There is a close relationship between increased NCDR in areas with low human development index (HDI) scores, which encompasses three metrics; life expectancy at birth, level of education and per capita income. It follows that leprosy NCDR are highest in states with low HDI scores including Brazilian states in the central-west (Goias, Mato Grosso) and north (Pará, Maranhão, Tocantins). Rates vary in these states from very high to hyperendemic, between 2 and >4 new cases per 10,000 population (similar to rates found in Norway in the latter part of the 19th century). By contrast, rates are ten-fold lower or less in states with a high HDI, such as the Federal District and southern states. Meta-analysis of secondary data links poor socioeconomic conditions with being at risk for leprosy with food deprivation, low education levels and low income being most relevant 138, 139. Other documented risk factors include living in an area highly endemic for leprosy exposure, overcrowding within the household, lack of access to health care, lack of clean drinking water causing bacterial, amoebal or helminth co-infections; the latter that can cause a shift to a Th2-ineffective immune response for leprosy140. More recent data suggest a decline in the numbers of new cases in children, with a concurrent rise in cases in elderly individuals over the age of 65, particularly in southern Brazilian states, like Rio Grande do Sul where leprosy rates have fallen to below 0.1 new case per 10,000 population 141, 142. Thus, the southern states are trending towards leprosy elimination similar to the decline in Norway observed at the turn of the 20th century.
VIII-c. Leprosy in the United States of America.
In the US, 1801 new leprosy cases were diagnosed at National Hansen’s Disease Program (NHDP) between 2011 – 2020. The US generally followed the global trend in male to female patient ratio (2:1) and that of multibacillary (~60%) to paucibacillary (~40%) cases. Thirty-one cases were positive for M. lepromatosis during 2013 – 2020 of which 10 cases had co-infection with M. leprae. The majority of patients (>50%) were above the age of 45 years at the time of diagnosis. Patients are routinely screened for different biopsychosocial determinants, and most patients showed elevated scores in at least one of these determinants immediately following diagnosis. Moreover, in most patients’ depression and anxiety scores were elevated following an episode of reaction, onset or deterioration of neuropathy, and drug-induced side effects, especially hyperpigmentation caused by clofazimine. Many US patients also have comorbidities, mainly diabetes, which becomes a challenge during leprosy reactions as prolonged corticosteroid is still the mainstay of reaction treatment. This clearly shows a need for a better treatment regimen along with therapeutic intervention(s) to prevent or manage reactions and nerve damage. Over the years, we have developed various clinical and sub-clinical mouse models, together with various biochemical and molecular methods to determine viability of the uncultivable M. leprae 143, 144. Leveraging these tools, we have now developed a drug evaluation pipeline to evaluate the efficacy of any bactericidal drug or drug combination regimen against both M. leprae and M. lepromatosis. We have used this pipeline to evaluate more than 30 anti-mycobacterial candidates that are in different stages of clinical development 47, 145. Telacebec (Q203), Macozinone (PBTZ-169), and Sutezolid are some of the most promising ones and are currently being tested in combination with other drugs to determine whether an effective shorter intermittent treatment regimen is possible.
VIII-d. Clinical experience in treating patients with leprosy in the U.S., Mexico, Colombia, and Cuba.
Neglected tropical diseases (NTDs) are remediable injustices of our times. Poverty and social exclusion represent the starting point, and the ultimate outcome of this group of diseases includes leprosy. In the context of NTDs, poverty should be seen as the relative deprivation of freedoms and capabilities dictating a lack of opportunities and choices in life to accomplish what we value as human beings 146. Leprosy is a disease that predominantly affects socially excluded populations and that promotes poverty by relatively depriving individuals of basic capabilities and freedoms. The social pathways of becoming ill with leprosy include socially determined failures, including widespread illiteracy, malnutrition, poor living conditions, unemployment, and the overall failure of ownership relations in the form of entitlements. In turn, in a vicious cycle of destitution and dispossession, leprosy and other NTDs produce disability, disfigurement, stigma, and premature mortality 147.
The persistent transmission of leprosy in many resource-poor settings in the US, Mexico, Colombia, and Cuba—despite the widespread deployment of multidrug therapy—represents an indicator of a world where many human groups live with limited access to education, sanitation, health, and social services. All of these factors represent the result of historical legacies that result in social injury. Leprosy does not occur in an ecological and societal vacuum, and thus, a vertical neoliberal approach to control or eliminate the disease is insufficient. Leprosy is closely related to conditions of poverty and maldistribution of wealth, power structures, and different valuation of human lives 148. Achieving a leprosy free world requires performing social autopsies, which are assessments to highlight the importance of social and non-biological forces contributing to the continuous occurrence of leprosy and its sequelae “beyond the numbers”, to implement comprehensive horizontal interventions that invest in human and social capital and the overall well-being of the affected individuals and communities. Optimally, social improvements to reduce inequities are prerequisites to reduce the prevailing unfair social arrangements conducive to the persistent occurrence of leprosy and other neglected infectious diseases. Leprosy is an ancient disease that offers a window of opportunity to learn the ecological expression of social inequalities and the historical power of stigma but also of the endurance of the human spirit 146, 148 (FIG 8).
VIII-e. The Armadillo model for leprosy neuropathy.
The nine-banded armadillo (Dasypus novemcinctus) is the only animal in North and South America, besides humans, that is naturally infected with M. leprae. Armadillos, like humans, exhibit the full clinical spectrum of leprosy and more importantly, they show extensive involvement of the peripheral nerves, and are recognized as a reliable model of leprosy neuritis. We developed, standardized, and validated a variety of invasive and non-invasive tests to compare peripheral nerve functions between uninfected and M. leprae-infected armadillos 149. We used these non-invasive tools to evaluate the efficacy of two potential neuro-protective drugs, ethoxyquin (EQ) and 4-aminopyridine (4-AP) in their ability to ameliorate peripheral nerve injury in M. leprae-infected armadillos. 4-AP treatment improved motor nerve conduction velocity (MNCV), compound muscle action potential (cMAP), and epidermal nerve fiber density (ENFD) compared to untreated animals, while EQ did not have any significant effect. These non-invasive tools have made it possible for us to do routine longitudinal follow-up of infected armadillos for nerve function impairment (NFI) which revealed that onset of NFI is early (~4-6 months post-inoculation) in these animals 149, 150. These tools have also enabled us to identify infected armadillos that are likely exhibiting pure neuronal form of leprosy. These animals are classified as pure neuronal when they show NFI in the absence of anti-PGL-1 positivity for the entire course of the disease (usually 18-24 months) and full dissemination of M. leprae infection as indicated by extremely low bacterial counts in the internal organs (liver and spleen) but higher counts in peripheral nerves. These results support the armadillo as a model for M. leprae-induced peripheral nerve injury that can provide insights toward the understanding of NFI progression and contribute to the preclinical investigation of the safety and efficacy of neuro-preventive and neuro-therapeutic interventions for leprosy.
VIII-f. Blood transcriptome in leprosy reversal reaction.
One in three people with leprosy develop a pathologic immune reaction, reversal reaction or erythema nodosum leprosum, which causes significant additional morbidity 151. Delay in recognition and treatment of immune reactions increases the risk of irreversible damage related to neuritis. Treatment of the reactions is with high-dose corticosteroids or potentially toxic immune regulators such as thalidomide. Dr. Dupnik described studies done with the Federal University of Rio Grande do Norte in Brazil to characterize differential gene expression in peripheral blood mononuclear cells (PBMC) during reversal reactions 152, including preliminary, unpublished data from a study of the transcriptome of paired PBMC from four people prior to and at the onset of reversal reaction. These data were additional support for systemic immunologic changes during reversal reactions.
IX. BOVINE TUBERCULOSIS AND ITS ENVIRONMENTAL CONFOUNDERS
In addition to confounders that originate within the host (e.g. disease comorbidities) or from other microbes (e.g. coinfections), other mycobacterial disease confounders originate in the environment; these include air pollution, climate change, and human encroachment on wildlife reservoirs of mycobacterial pathogens. The discussion of environmental confounders is particularly relevant to bovine TB, zoonotic TB (zTB), and the One Health approach that recognizes the interconnection between people, animals, plants, and their shared environment in achieving optimal heath. Addressing these topics were Dr. Liliana Salvador (University of Arizona), Dr. Sandrine Lesellier (ANSES, France), Dr. Paola Boggiatto (USDA), Dr. Claudia Perea (USDA), and Dr. James Sunstrum (Beaumont Health). Summaries of select talks are provided below.
IX-a. M. bovis genomic signatures across organizational scales.
M. bovis, the causative agent of bovine tuberculosis (bTB), infects a broad mammalian host range (including humans), creating opportunities for cross-species transmission at the wildlife-livestock interface, which impacts the success of bTB control measures worldwide. M. bovis is considered a generalist pathogen, however, only a few mammal species become reservoirs of infection after spillover events, and the ability to identify host-species that have the potential to maintain and transmit pathogens and/or regions that have high risk of becoming endemic, would improve current surveillance and control strategies. To determine potential adaptation mechanisms to different hosts and/or regions, Legall and Salvador 153 identified M. bovis genomic signatures that are associated with these organization scales by performing a comparative genomic analysis on publicly available datasets of M. bovis collected from three bTB endemic regions (United Kingdom 154, New Zealand 155, and United States 156). These datasets collectively included a total of 11 host species with a balanced combination of wild and domestic hosts 153. M. bovis genomic regions that contained evolutionary signatures such as selective sweep regions and high Single Nucleotide Polymorphisms (SNPs) density regions were identified and used in random forest models to determine if they were predictive of M. bovis endemic regions and host-species. The genes (and their associated functions) related to these genomic regions were identified and further analyzed to study their involvement in M. bovis transmission and persistence in different environments. The 132 selective sweep and 14 high SNP density regions that were found predicted with high accuracy (≥0.945) the country of origin and the UK host-species (badgers vs cattle). The top two predictors for host species in the UK were one particular selective sweep region and one particular high SNP density region that contain genes that are implicated in mycobacterial adaptation (vapb18 and vapc18) as well as lipoproteins that affect lipid modifications (lppA, lppB, plrR). High rate of mutations in these genes could be associated with adaptation to the immunological pressures within certain hosts and a close monitoring of these regions could provide a signal that M. bovis is persisting within a population.
IX-b. Bovine TB in badgers.
Bovine TB caused by M. bovis remains one of the most important animal diseases worldwide and an underestimated burden of tuberculosis in humans. In Europe, the TB prevalence of TB can exceed 10% of herds in the UK and it can be challenging to maintain prevalence below 0.1% in Officially TB free countries) with significant financial, social and environmental impacts. TB eradication in cattle is therefore urgent with control of infection from all main sources, including wildlife reservoirs for TB. Developing oral veterinary vaccines for badgers (Meles meles) against TB 157, 158 is therefore an international endeavor to sustainably reduce the long-term risks of transmission to cattle. The BCG vaccine, as well as M. bovis, stimulates strong Th1 responses in badgers against M. bovis antigens (bovine tuberculin - Purified Protein Derivative-B PPD-B), but also to M. avium tuberculin (PPD-A), an important environmental mycobacteria that also contaminate badgers 159, 160.
Pre-infection with M. avium tends to be associated with more severe TB disease in experimentally infected badgers 161, and may also impact the response of wild badgers to the BCG vaccine. Ferrets are laboratory animal models for screening vaccines before testing in badgers and generally do not respond to PPD-A, suggesting they are not exposed to M. avium. In parallel, badgers responding to PPD-B but also A generally do not grant a response against ESAT-6 162, while TB infected ferrets (always PPD-A negative) do. This different mycobacterial pre-exposure levels and background immune responses between ferrets and badgers should be taken in account when testing the protective efficacy provided by oral vaccine candidates in mustelids, as factors that may influence severity scores and for immune responses.
IX-c. Oral vaccination against bovine tuberculosis for free-ranging white-tailed deer.
Bovine TB, caused by infection with M. bovis has been estimated to affect >50 million cattle with an economic impact of $3 billion annually. The implementation of national eradication program has been successful in decreasing disease prevalence, yet eradication remains elusive. One obstacle to eradication is the presence of wildlife reservoirs of M. bovis. In the United States, white-tailed deer were identified as the reservoir for disease in Michigan. Since then, spill back onto cattle herds has been an ongoing issue in this state, where the focus of infected white-tailed deer remains. Surveillance and control measures were implemented to control bTB spread in free-ranging white-tailed deer populations. While successful in decreasing disease prevalence, eradication has not been achieved. Vaccination of white-tailed deer has been proposed as an additional method to help control disease spread. In several penned deer studies, oral administration of liquid BCG was shown to be protective against virulent M. bovis challenge. Protection was defined as a reduced number of lesions, fewer advanced grade granulomas, and decreased dissemination to thoracic and extra-thoracic lymph nodes, when compared to non-vaccinated animals. Altogether, these features could translate into a decreased potential for disease spread, and therefore, aid in disease control. However, vaccination of free-ranging wildlife presents its own unique set of challenges including adequate administration platforms, ensuring vaccine availability, and measuring vaccine efficacy.
IX-d. World Mycobacterium bovis project.
M. bovis causes tuberculosis in livestock and wildlife worldwide. Furthermore, it also causes disease in humans, making it an important zoonotic pathogen where animal tuberculosis is endemic. Presently, whole genome sequencing can provide a great benefit to disease investigations and research. The National Veterinary Services Laboratories of the USDA has an ongoing “World M. bovis Project” aimed at characterizing M. bovis strains from across the world to improve source attribution both locally and internationally. Ultimately, the goal is to develop a curated database of M. bovis genomes that includes associated metadata that can be used to provide a more comprehensive phylogenetic analysis of M. bovis lineages and that is representative of its overall diversity. Collaborations have already been established with more than 35 countries, including representatives of the animal health authorities (national reference laboratories) and researchers from each country. Currently, a total of 39 main phylogenetic groups have been identified, which represent the main clonal complexes described for M. bovis: European 1, European 2, European 3 (“BCG-like”), African 1 and African 2. Lineages that do not fall within these complexes have also been identified. Overall, the observed distribution of the genetic diversity reflects the patterns of natural livestock migration and historical and recent commercial trade routes for cattle. Interestingly, human migration may also be playing a role in the worldwide distribution of M. bovis.
IX-e. Zoonotic M. bovis disease in patients with exposure to wild deer – Michigan 2002-2022.
Bovine TB was thought to have been eliminated from cattle in Michigan in the 1980’s. This was accomplished by decades of veterinary work to test cattle with skin tests, slaughter cattle with reactive skin tests, and quarantine of affected herds. This program significantly reduced the amount of human disease from M. bovis, as less contaminated milk and dairy products were consumed. However, there was an unrecognized transmission of the infection from cattle into free-ranging deer in a small area of NE Michigan, which was recognized in 1994. This is now identified as a chronic enzoonotic focus of infection, involving deer and spreading back into cattle herds. Since the discovery of the spillback into cattle, over 88 herds have been affected causing significant economic problems for cattle producers. Surveillance of hunted deer for diseased lymph nodes has identified over 900 infected deer. Since 2002 seven humans have been diagnosed with illness due to M. bovis, and data suggest they became infected following exposure to diseased deer. Five human cases involved the pulmonary system, and two cases sustained a finger injury handling a carcass triggering finger infections (FIG 9). Zoonotic TB needs further research into the epidemiology of human exposure, risk assessment, and mitigation of animal reservoirs of disease. A One Health approach to deal with this enzoonotic focus involves veterinarians examining both cattle and wildlife, and close collaboration with public health physicians. Working on this problem may serve as a management model to provide key insights into zTB transmission in other regions of the world, as zTB is increasingly being recognized as a global health issue.
FIGURE 9. Tuberculosis among white-tailed deer in Michigan.
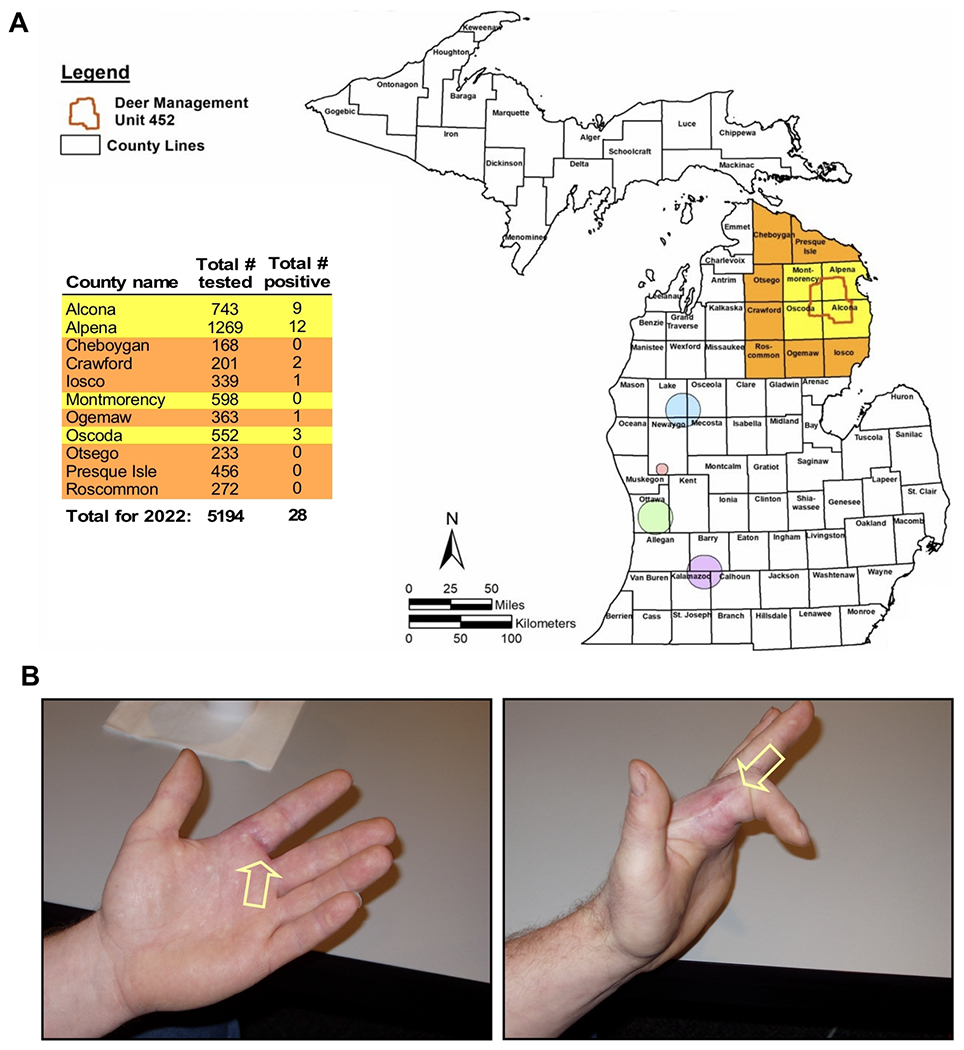
(A) Deer Management Unit (DMU) 452 is a large area in Michigan, USA, where bovine TB is endemic in white-tailed deer (Odocoileus virginianus). Shown is the location DMU 452 in relation to the counties surrounding it, which are subjected to both active and passive surveillance by the Michigan Department of Natural Resources (DNR), as part of their disease mitigation efforts to prevent spillover to cattle. For active surveillance, hunters voluntarily submit the heads of deer for examination; for passive surveillance, hunters may submit deer carcasses with TB chest lesions from anywhere in the state. In yellow and orange are counties surrounding DMU 452, where surveillance efforts are most focused, as well as distal testing sites (circled) to surveille bovine TB movement south (the Great Lakes prevent bovine TB movement north). The table inset lists for each county surrounding DMU 452 the total number of deer tested and total positive for M. bovis in 2022, as originally reported by the Michigan DNR. (B) An M. bovis-infected hand, the result of a zoonotic transmission event which occurred while preparing an M. bovis-infected deer head for taxidermy. Images courtesy of James Sunstrum.
X. RECENT INNOVATIONS IN MYCOBACTERIAL RESEARCH
The final panel discussion of MHM9 was dedicated to advances in a variety of fields, highlighting the breadth of approaches being taken to identify host and microbial factors contributing to mycobacterial disease pathogenesis and transmission. As led by Dr. Karen Lacourciere (NIAID), panelists included Dr. Igor Kramnik (Boston University), Dr. Gillian Beamer (Texas Biomedical Research Institute), Dr. Keith Derbyshire (The Wadsworth Institute), Dr. Srinand Sreevatsan (Michigan State University), Dr. David Tobin (Duke University), and Dr. Charlie Pyle (Duke University), who respectively presented on their work regarding a mouse model of post-primary pulmonary TB 163, advances in artificial intelligence-assisted histological analysis 164, 165, exploiting the genetic orthologs of M. smegmatis to understand mycobacterial pathogenesis 166, 167, whole-genome analysis of Mtb isolates from Asian elephants of Nepal 168, and mycobacteria evolution within the granuloma. Summaries of these presentations are provided below.
X-a. A mouse model of post-primary pulmonary TB.
Post-primary pulmonary TB (pppTB) is a major clinical form of tuberculosis in adult patients. It has been shown that in the majority of cases the pppTB develops as a result of reactivation and dissemination of Mtb from primary lesions to the upper lung lobes. There, the secondary lesions may progress to form necrotic cavitary lesions. It remains unknown which factors are involved in the formation and propagation of secondary lung lesions and why systemic immunity fails to control the pulmonary TB progression. The Kramnik lab reported the development of a mouse model of pppTB in immunocompetent mice. This model uses a mouse strain that carries the sst1 TB susceptibility locus on the B6 genetic background and has been shown to develop organized necrotic granulomas specifically in the lungs after aerosol infections with virulent Mtb. In the pppTB model, virulent Mtb is not delivered directly to the lungs via aerosols, but the primary lesion is created remotely by injecting Mtb in a mouse hock. The dissemination of Mtb to and persistence in the regional lymph nodes, spleens and other parenchymal organs was observed in all animals for up to 6 months of infection. However, only in the lungs the macroscopic TB lesions were formed and progressed toward the necrotization. The pulmonary lesion progression was monitored using acid fast staining, multiplexed fluorescent immunostaining and spatial transcriptomics. Their preliminary results demonstrated that the pulmonary lesions progressed in the sst1-susceptible B6. Sst1S mice, but not in their B6 wild type counterparts, within 3 - 6 months post infection from the interstitial paucibacillary granulomatous lesions to multibacillary pneumonic lesions with areas of necrotic inflammation (FIG 10). The loss of Mtb control was associated with an aberrant macrophage activation within pulmonary TB lesions that was characterized by the hyperactivity of type I interferon pathway and the appearance and expansion of an Arg1+ macrophage population. Providing further insights into specific mechanisms of pulmonary TB progression, this model may also be useful for pre-clinical testing of post-exposure interventions.
FIGURE 10. Progression of post-primary pulmonary TB lesions is associated with macrophage polarization.
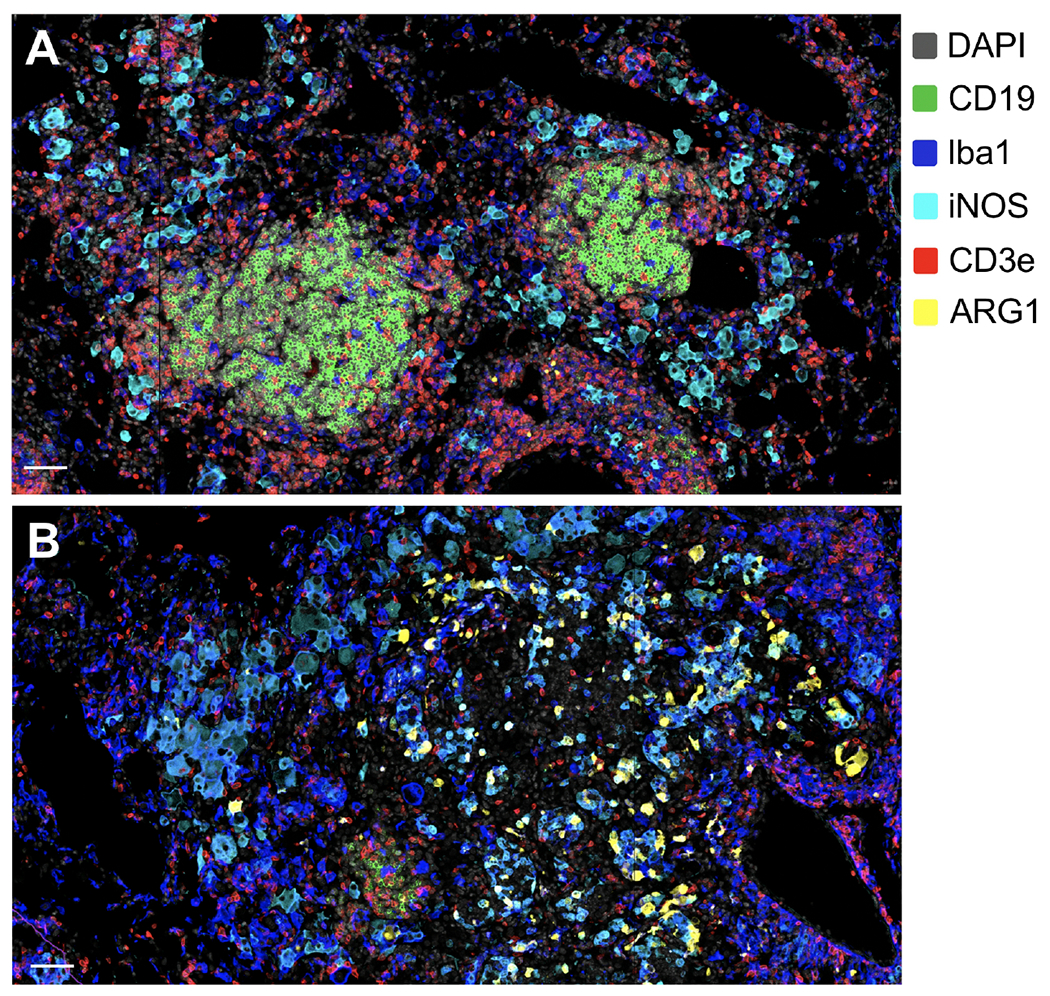
Post-primary pulmonary TB lesions in B6.Sst1S mice 14 weeks after footpad infection with 106 of virulent Mtb Erdman. Shown are (A) a controlling paucibacillary lesion, and (B) a non-controlling multibacillary lesion, with stains corresponding to T lymphocytes (CD3+, red), B lymphocytes (CD19+, green), and macrophages (Iba1+, blue) expressing nitric oxide synthase (iNOS+, magenta) or arginase (Arg1+, yellow). Scale bare = 50 microns. Acid fast staining on serial sections was used to classify TB lesions: controlling lesions were paucibacillary and Mtb was intracellular with 1-2 bacilli per infected macrophage. The non-controlling lesions were multibacillary with clusters of intracellular Mtb and areas of micronecrosis containing extracellular bacteria. Multiplexed fluorescent immunohistochemistry and quantification using HALO software were performed as described in Rosenbloom et al 178. Images courtesy of Igor Kramnik and Nicholas Crossland.
X-b. Artificial intelligence assisted histological analysis.
The complexity, dynamic nature and importance of TB granuloma has been recently reviewed 169. A perennial challenge for TB researchers across the career spectrum is how to best derive meaningful, quantitative data from post-mortem TB granuloma visualizations. TB granulomas are typically visualized by staining sections of granulomatous tissues with dye combinations such hematoxylin and eosin (H&E, to visualize general structure), carbol fuchsin with methylene blue counterstain (to visualize AFB), or more specialized immunohistochemistry reagents to identify specific cell subsets. To generate meaningful, quantitative data from these images, researchers must currently rely on pathologists to examine tissues and create de novo scoring systems or count cells/lesions across hundreds or thousands of sections. Pathologists are not always available to provide this service, which is necessarily slow and tedious, especially in resource-limited settings or institutions which do not have clinical pathology service lines. To improve access to pathology expertise, as well as improve the efficiency and reproducibility across experiments (and investigators) while also reducing bias, the Beamer lab and a commercial partner are developing an automated, artificial intelligence (AI) based tool for extracting complex visual information from TB granulomas. Beamer presented on her progress building and validating AI modules for lung sections dual-stained for H&E plus AFB, the most common stains in TB research, to detect and quantify features (e.g., necrosis, immune and inflammatory cells, bacteria, etc.) within TB granulomas to gain insight into spatial relationships, and functional outcomes. MHM9 participants were invited to contribute specimens for their future endeavors focused on (i) AI modules for other stains, i.e., fluorescent products and immunohistochemical substrates; and (ii) use the AI maps to guide laser capture microdissection of regions of interest. Their hope is that by coupling these two technologies will support hypothesis driven research and support discovery to elucidate hidden structure-function relationships, as visually similar cellular regions in granulomas may have different gene expression profiles, and different protein expression 170, 171. It is anticipated that development, validation, testing, and access to this tool will accelerate our understanding of TB granulomas, and ultimately leading to better treatments and novel vaccines for TB.
X-c. Exploiting the genetic orthologs of M. smegmatis to understand mycobacterial pathogenesis.
Derbyshire discussed the establishment of a Mycobacterial Systems Resource (MSR) using the model organism M. smegmatis 166, 167. This resource focuses specifically on 1,153 highly conserved core genes that are common to most mycobacterial species including Mtb, M. leprae, M. abscessus, and M. marinum and, therefore, the encoded proteins likely perform conserved mycobacterial core functions. Thus, functional insights gained from the MSR will apply to all mycobacterial species including the less experimentally tractable pathogens. The biological resource includes (i) an expression-plasmid library of 1,116 genes fused to a fluorescent protein for determining protein localization, (ii) a library of 569 precise-deletions of non-essential genes, and (iii) a set of 843 CRISPR-interference plasmids specifically targeted to silence expression of essential core genes and genes for which a precise deletion was not obtained. The informatic resource includes information about individual genes and a detailed assessment of protein localization and is described at referenced websites 172, 173. Examples were presented on how the library and data sets can be utilized to identify genes that impact biofilm formation, proteins that co-localize within a cell and, therefore, are likely part of the same protein machinery (guilt by association), and how CRISPRi silencing can be exploited to identify conditionally essential genes. By selecting the most conserved genes that mediate core functions, the resource provides an experimental foundation to verify existing putative functional annotations or initially assign function to genes that currently lack direct empirical support. Most importantly for this research community, the MSR is the only centralized mutant or cloned gene resource for all Mycobacterium Sps, despite the recognized impact of NTMs on global health.
X-d. Mycobacteria evolution within the granuloma.
Granulomas are evolutionarily conserved, multilayer immune complexes, that can isolate pathogenic mycobacteria within their hosts. Using multiple model systems, including zebrafish, we showed that during mycobacterial infection, granuloma macrophages undergo epithelioid transformation events that contribute to granuloma formation and stability. stat6-deficient macrophages fail to undergo epithelioid transformation, leading to an absence of structured granulomas 174. Other discrete granuloma macrophage subsets and cell-specific pathways appear to be uniquely responsible for containment of the granuloma’s bacteria-rich necrotic core. In addition to epithelioid macrophage transformation, pathogenic mycobacteria can regulate the motility of infected macrophages to influence dissemination. We identified EsxM as an ancestral bacterial secreted effector that contributes to increased rates of dissemination in “ancestral-lineage” strains of Mtb 175. The studies we presented highlight the co-evolution of pathways in both host and pathogen that reprogram macrophages to enhance regional consolidation of mycobacterial lesions.
XI. REMEMBERING THOSE WHO HAVE PASSED
As in previous MHM meetings 176, time was taken to remember those who passed on after having made important contributions to our understanding of mycobacterial disease and/or care of people affected by mycobacterial disease. Remembered individuals include Dr. Susan La Flesche Picotte. A member of the Omaha people, Dr. Picotte was the first Indigenous person to be conferred with a medical degree in the Americas. For many years she was the only medical doctor for ~1200 Omaha people over an area of 1300 square miles. She cared for many individuals with TB and petitioned the US federal government for aid to establish a hospital on the Omaha reservation; receiving none, she led the efforts that established a privately run hospital for both Indigenous and non-Indigenous people alike 21. Her hospital is currently being restored as a community center and wellness clinic in her honor 177.
ACKNOWLEDGEMENTS
We would like to acknowledge the land that The Ohio State University (OSU) occupies is the ancestral and contemporary territory of the Shawnee, Potawatomi, Delaware, Miami, Peoria, Seneca, Wyandotte, Ojibwe, and many other Indigenous peoples. As a land grant institution, we want to honor the resiliency of these tribal nations and recognize the historical contexts that have and continue to affect the Indigenous peoples of this land. The Many Hosts of Mycobacteria 9 conference was supported by the OSU Infectious Disease Institute (IDI), OSU Global One Health Initiative (GoHi), and National Institute of Health (NIH)/National Institute of Allergy and Infectious Diseases (NIAID) grant 1R13AI169929-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
BIBLIOGRAPHY
- 1.Schildknecht KR, Pratt RH, Feng PI, Price SF, Self JL. Tuberculosis - United States, 2022. MMWR Morb Mortal Wkly Rep 2023;72:297–303. doi: 10.15585/mmwr.mm7212a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ehrenpreis JE, Ehrenpreis ED. A Historical Perspective of Healthcare Disparity and Infectious Disease in the Native American Population. Am J Med Sci 2022;363:288–294. doi: 10.1016/j.amjms.2022.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bloss E, Holtz TH, Jereb J, Redd JT, Podewils LJ, Cheek JE, McCray E. Tuberculosis in indigenous peoples in the U.S., 2003-2008. Public Health Rep 2011;126:677–689. doi: 10.1177/003335491112600510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daniel TM. The history of tuberculosis. Respir Med 2006;100:1862–1870. doi: 10.1016/j.rmed.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Gagneux S. Host-pathogen coevolution in human tuberculosis. Philos Trans R Soc Lond B Biol Sci 2012;367:850–859. doi: 10.1098/rstb.2011.0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lonnroth K, Raviglione M. Global epidemiology of tuberculosis: prospects for control. Semin Respir Crit Care Med 2008;29:481–491. doi: 10.1055/s-0028-1085700. [DOI] [PubMed] [Google Scholar]
- 7.Pepperell CS, Granka JM, Alexander DC, Behr MA, Chui L, Gordon J, Guthrie JL, Jamieson FB, Langlois-Klassen D, Long R, Nguyen D, Wobeser W, Feldman MW. Dispersal of Mycobacterium tuberculosis via the Canadian fur trade. Proc Natl Acad Sci U S A 2011;108:6526–6531. doi: 10.1073/pnas.1016708108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roberts CA, Buikstra JE. The bioarchaeology of tuberculosis: a global perspective on a re-emerging disease. University Press of Florida; 2003 [Google Scholar]
- 9.Allison MJ, Gerzten E, Munizaga JH, Santoro C, Mendoza D. Tuberculosis in pre-Columbian Andean populations. In: Buikstra JE (Ed.), Prehistoric Tuberculosis in the Americas. Northwestern University Archeological Program, Evanston: 1981:49–62. [Google Scholar]
- 10.Lombardi Alonacin GP. Autopsia de una Momia de la Cultura Nasca: Es- tudio paleopatologico. . Universidad Peruana Cayetano Heredia: Tesis para optar el titulo de Medico Cirujano 1992 [Google Scholar]
- 11.Vagene AJ, Honap TP, Harkins KM, Rosenberg MS, Giffin K, Cardenas-Arroyo F, Leguizamon LP, Arnett J, Buikstra JE, Herbig A, Krause J, Stone AC, Bos KI. Geographically dispersed zoonotic tuberculosis in pre-contact South American human populations. Nat Commun 2022;13:1195. doi: 10.1038/s41467-022-28562-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bos KI, Harkins KM, Herbig A, Coscolla M, Weber N, Comas I, Forrest SA, Bryant JM, Harris SR, Schuenemann VJ, Campbell TJ, Majander K, Wilbur AK, Guichon RA, Wolfe Steadman DL, Cook DC, Niemann S, Behr MA, Zumarraga M, Bastida R, Huson D, Nieselt K, Young D, Parkhill J, Buikstra JE, Gagneux S, Stone AC, Krause J. Pre-Columbian mycobacterial genomes reveal seals as a source of New World human tuberculosis. Nature 2014;514:494–497. doi: 10.1038/nature13591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brites D, Loiseau C, Menardo F, Borrell S, Boniotti MB, Warren R, Dippenaar A, Parsons SDC, Beisel C, Behr MA, Fyfe JA, Coscolla M, Gagneux S. A New Phylogenetic Framework for the Animal-Adapted Mycobacterium tuberculosis Complex. Front Microbiol 2018;9:2820. doi: 10.3389/fmicb.2018.02820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gagneux S, DeRiemer K, Van T, Kato-Maeda M, de Jong BC, Narayanan S, Nicol M, Niemann S, Kremer K, Gutierrez MC, Hilty M, Hopewell PC, Small PM. Variable host-pathogen compatibility in Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 2006;103:2869–2873. doi: 10.1073/pnas.0511240103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levy S. The Evolution of Tuberculosis: Genetic analysis offers new insight on the spread of an ancient disease. BioScience 2012;62:625–629. doi: 10.1525/bio.2012.62.7.3. [DOI] [Google Scholar]
- 16.Heffernan C, Ferrara G, Long R. Reflecting on the relationship between residential schools and TB in Canada. Int J Tuberc Lung Dis 2022;26:811–813. doi: 10.5588/ijtld.22.0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trafzer CE. Tuberculosis death and survival among Southern California Indians, 1922-44. Can Bull Med Hist 2001;18:85–107. doi: 10.3138/cbmh.18.1.85. [DOI] [PubMed] [Google Scholar]
- 18.Rieder HL. Tuberculosis among American Indians of the contiguous United States. Public Health Rep 1989;104:653–657. [PMC free article] [PubMed] [Google Scholar]
- 19.Aronson JD, Palmer CE. Experience with BCB vaccine in the control of tuberculosis among North American Indians. Public Health Rep (1896) 1946;61:802–820. [PubMed] [Google Scholar]
- 20.Townsend JG, Aronson JD, Saylor R, Parr I. Tuberculosis control among the North American Indians. American Review of Tuberculosis 1942;45:41–52. [Google Scholar]
- 21.Ferry G Susan La Flesche Picotte: a doctor who spanned two cultures. Lancet 2019;393:734. doi: 10.1016/S0140-6736(19)30363-0. [DOI] [PubMed] [Google Scholar]
- 22.Lee NR, Winer RL, Cherne S, Noonan CJ, Nelson L, Gonzales AA, Umans JG, Buchwald D, Collaborative to Improve Native Cancer O. Human Papillomavirus Prevalence Among American Indian Women of the Great Plains. J Infect Dis 2019;219:908–915. doi: 10.1093/infdis/jiy600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee NR, Noonan CJ, Nelson L, Umans JG. HPV Knowledge and Attitudes Among American Indian and Alaska Native Health and STEM Conference Attendees. Int J Indig Health 2019;14:205–221. doi: 10.32799/ijih.v14i2.31920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bordeaux SJ, Baca AW, Begay RL, Gachupin FC, Caporaso JG, Herbst-Kralovetz MM, Lee NR. Designing Inclusive HPV Cancer Vaccines and Increasing Uptake among Native Americans-A Cultural Perspective Review. Curr Oncol 2021;28:3705–3716. doi: 10.3390/curroncol28050316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Desai AN, Seshasayee SM, Majumder MS, Lassmann B, Madoff LC, Cohn EL, Brownstein JS. Tuberculosis and foreign-born populations in the United States: A mixed methods pilot study of media reporting and political identification. PLoS One 2020;15:e0230967. doi: 10.1371/journal.pone.0230967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Courtwright A, Turner AN. Tuberculosis and stigmatization: pathways and interventions. Public Health Rep 2010;125 Suppl 4:34–42. doi: 10.1177/00333549101250S407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Comas I, Coscolla M, Luo T, Borrell S, Holt KE, Kato-Maeda M, Parkhill J, Malla B, Berg S, Thwaites G, Yeboah-Manu D, Bothamley G, Mei J, Wei L, Bentley S, Harris SR, Niemann S, Diel R, Aseffa A, Gao Q, Young D, Gagneux S. Out-of-Africa migration and Neolithic coexpansion of Mycobacterium tuberculosis with modern humans. Nat Genet 2013;45:1176–1182. doi: 10.1038/ng.2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weiss ES. Tuberculin sensitivity in Alaska. Public Health Rep (1896) 1953;68:23–27. [PMC free article] [PubMed] [Google Scholar]
- 29.Kaplan GJ, Fraser RI, Comstock GW. Tuberculosis in Alaska, 1970. The continued decline of the tuberculosis epidemic. Am Rev Respir Dis 1972;105:920–926. doi: 10.1164/arrd.1972.105.6.920. [DOI] [PubMed] [Google Scholar]
- 30.Comstock GW, Philip RN. Decline of the tuberculosis epidemic in Alaska. Public Health Rep (1896) 1961;76:19–24. [PMC free article] [PubMed] [Google Scholar]
- 31.Comstock GW, Baum C, Snider DE Jr. Isoniazid prophylaxis among Alaskan Eskimos: a final report of the bethel isoniazid studies. Am Rev Respir Dis 1979;119:827–830. doi: 10.1164/arrd.1979.119.5.827. [DOI] [PubMed] [Google Scholar]
- 32.Comstock GW, Ferebee SH, Hammes LM. A controlled trial of community-wide isoniazid prophylaxis in Alaska. Am Rev Respir Dis 1967;95:935–943. doi: 10.1164/arrd.1967.95.6.935. [DOI] [PubMed] [Google Scholar]
- 33. http://www.epi.alaska.gov/bulletins/docs/b2023_01.pdf .
- 34.Walsh CM, Gebert MJ, Delgado-Baquerizo M, Maestre FT, Fierer N. A Global Survey of Mycobacterial Diversity in Soil. Appl Environ Microbiol 2019;85 doi: 10.1128/AEM.01180-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gebert MJ, Delgado-Baquerizo M, Oliverio AM, Webster TM, Nichols LM, Honda JR, Chan ED, Adjemian J, Dunn RR, Fierer N. Ecological Analyses of Mycobacteria in Showerhead Biofilms and Their Relevance to Human Health. mBio 2018;9 doi: 10.1128/mBio.01614-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Komm O, Almeida DV, Converse PJ, Omansen TF, Nuermberger EL. Impact of Dose, Duration, and Immune Status on Efficacy of Ultrashort Telacebec Regimens in Mouse Models of Buruli Ulcer. Antimicrob Agents Chemother 2021;65:e0141821. doi: 10.1128/AAC.01418-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Almeida DV, Converse PJ, Omansen TF, Tyagi S, Tasneen R, Kim J, Nuermberger EL. Telacebec for Ultrashort Treatment of Buruli Ulcer in a Mouse Model. Antimicrob Agents Chemother 2020;64 doi: 10.1128/AAC.00259-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Converse PJ, Almeida DV, Tyagi S, Xu J, Nuermberger EL. Shortening Buruli Ulcer Treatment with Combination Therapy Targeting the Respiratory Chain and Exploiting Mycobacterium ulcerans Gene Decay. Antimicrob Agents Chemother 2019;63 doi: 10.1128/AAC.00426-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scherr N, Bieri R, Thomas SS, Chauffour A, Kalia NP, Schneide P, Ruf MT, Lamelas A, Manimekalai MSS, Gruber G, Ishii N, Suzuki K, Tanner M, Moraski GC, Miller MJ, Witschel M, Jarlier V, Pluschke G, Pethe K. Targeting the Mycobacterium ulcerans cytochrome bc(1):aa(3) for the treatment of Buruli ulcer. Nat Commun 2018;9:5370. doi: 10.1038/s41467-018-07804-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaser M, Rondini S, Naegeli M, Stinear T, Portaels F, Certa U, Pluschke G. Evolution of two distinct phylogenetic lineages of the emerging human pathogen Mycobacterium ulcerans. BMC Evol Biol 2007;7:177. doi: 10.1186/1471-2148-7-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoshida M, Nakanaga K, Ogura Y, Toyoda A, Ooka T, Kazumi Y, Mitarai S, Ishii N, Hayashi T, Hoshino Y. Complete Genome Sequence of Mycobacterium ulcerans subsp. shinshuense. Genome Announc 2016;4 doi: 10.1128/genomeA.01050-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stinear TP, Seemann T, Pidot S, Frigui W, Reysset G, Garnier T, Meurice G, Simon D, Bouchier C, Ma L, Tichit M, Porter JL, Ryan J, Johnson PD, Davies JK, Jenkin GA, Small PL, Jones LM, Tekaia F, Laval F, Daffe M, Parkhill J, Cole ST. Reductive evolution and niche adaptation inferred from the genome of Mycobacterium ulcerans, the causative agent of Buruli ulcer. Genome Res 2007;17:192–200. doi: 10.1101/gr.5942807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Converse PJ, Almeida DV, Nuermberger EL, Grosset JH. BCG-mediated protection against Mycobacterium ulcerans infection in the mouse. PLoS Negl Trop Dis 2011;5:e985. doi: 10.1371/journal.pntd.0000985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Portaels F, Aguiar J, Debacker M, Steunou C, Zinsou C, Guedenon A, Meyers WM. Prophylactic effect of mycobacterium bovis BCG vaccination against osteomyelitis in children with Mycobacterium ulcerans disease (Buruli Ulcer). Clin Diagn Lab Immunol 2002;9:1389–1391. doi: 10.1128/cdli.9.6.1389-1391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zwerling A, Behr MA, Verma A, Brewer TF, Menzies D, Pai M. The BCG World Atlas: a database of global BCG vaccination policies and practices. PLoS Med 2011;8:e1001012. doi: 10.1371/journal.pmed.1001012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim J, Choi J, Kang H, Ahn J, Hutchings J, Niekerk CV, Kim J, Jeon Y, Nam K, Kim TH, Shin BS, Shin S. Safety, Tolerability, Pharmacokinetics, and Metabolism of Telacebec (Q203) for the Treatment of Tuberculosis: a Randomized, Placebo-Controlled, Multiple Ascending Dose Phase 1B Trial. Antimicrob Agents Chemother 2023;67:e0112322. doi: 10.1128/aac.01123-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lahiri R, Adams LB, Thomas SS, Pethe K. Sensitivity of Mycobacterium leprae to Telacebec. Emerg Infect Dis 2022;28:749–751. doi: 10.3201/eid2803.210394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roller M, Hansen S, Knauf-Witzens T, Oelemann WMR, Czerny CP, Abd El Wahed A, Goethe R. Mycobacterium avium Subspecies paratuberculosis Infection in Zoo Animals: A Review of Susceptibility and Disease Process. Front Vet Sci 2020;7:572724. doi: 10.3389/fvets.2020.572724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carta T, Alvarez J, Perez de la Lastra JM, Gortazar C. Wildlife and paratuberculosis: a review. Res Vet Sci 2013;94:191–197. doi: 10.1016/j.rvsc.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 50.Burgess TL, Witte CL, Rideout BA. Early-life exposures and Johne’s disease risk in zoo ruminants. J Vet Diagn Invest 2018;30:78–85. doi: 10.1177/1040638717735350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kimura K, Reinhardt TA, Goff JP. Parturition and hypocalcemia blunts calcium signals in immune cells of dairy cattle. J Dairy Sci 2006;89:2588–2595. doi: 10.3168/jds.S0022-0302(06)72335-9. [DOI] [PubMed] [Google Scholar]
- 52.Bernitz N, Kerr TJ, Goosen WJ, Chileshe J, Higgitt RL, Roos EO, Meiring C, Gumbo R, de Waal C, Clarke C, Smith K, Goldswain S, Sylvester TT, Kleynhans L, Dippenaar A, Buss PE, Cooper DV, Lyashchenko KP, Warren RM, van Helden PD, Parsons SDC, Miller MA. Review of Diagnostic Tests for Detection of Mycobacterium bovis Infection in South African Wildlife. Front Vet Sci 2021;8:588697. doi: 10.3389/fvets.2021.588697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O’Halloran C, Tornqvist-Johnsen C, Woods G, Mitchell J, Reed N, Burr P, Gascoyne-Binzi D, Wegg M, Beardall S, Hope J, Gunn-Moore D. Feline tuberculosis caused by Mycobacterium bovis infection of domestic UK cats associated with feeding a commercial raw food diet. Transbound Emerg Dis 2021;68:2308–2320. doi: 10.1111/tbed.13889. [DOI] [PubMed] [Google Scholar]
- 54.Meiring C, Higgitt R, Dippenaar A, Roos E, Buss P, Hewlet J, Cooper D, Rogers P, de Klerk-Lorist L, van Schalkwyk L, Hausler G, van Helden P, Moller M, Warren R, Miller M. Characterizing epidemiological and genotypic features of Mycobacterium bovis infection in wild dogs (Lycaon pictus). Transbound Emerg Dis 2021;68:3433–3442. doi: 10.1111/tbed.13947. [DOI] [Google Scholar]
- 55.Kerr TJ, Gumbo R, Goosen WJ, Rogers P, Last RD, Miller MA. Novel Techniques for Detection of Mycobacterium bovis Infection in a Cheetah. Emerg Infect Dis 2020;26:630–631. doi: 10.3201/eid2603.191542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Viljoen IM, van Helden PD, Millar RP. Mycobacterium bovis infection in the lion (Panthera leo): Current knowledge, conundrums and research challenges. Vet Microbiol 2015;177:252–260. doi: 10.1016/j.vetmic.2015.03.028. [DOI] [PubMed] [Google Scholar]
- 57.Miller M, Joubert J, Mathebula N, De Klerk-Lorist LM, Lyashchenko KP, Bengis R, van Helden P, Hofmeyr M, Olea-Popelka F, Greenwald R, Esfandiari J, Buss P. Detection of antibodies to tuberculosis antigens in free-ranging lions (Panthera leo) infected with Mycobacterium bovis in Kruger National Park, South Africa. J Zoo Wildl Med 2012;43:317–323. doi: 10.1638/2011-0171.1. [DOI] [PubMed] [Google Scholar]
- 58.Meiring C, Higgitt R, Goosen WJ, van Schalkwyk L, de Klerk-Lorist LM, Buss P, van Helden PD, Parsons SDC, Moller M, Miller M. Shedding of Mycobacterium bovis in respiratory secretions of free-ranging wild dogs (Lycaon pictus): Implications for intraspecies transmission. Transbound Emerg Dis 2021;68:2581–2588. doi: 10.1111/tbed.14125. [DOI] [PubMed] [Google Scholar]
- 59.Miller M, Buss P, Hofmeyr J, Olea-Popelka F, Parsons S, van Helden P. Antemortem diagnosis of Mycobacterium bovis infection in free-ranging African lions (Panthera leo) and implications for transmission. J Wildl Dis 2015;51:493–497. doi: 10.7589/2014-07-170. [DOI] [PubMed] [Google Scholar]
- 60.Higgitt RL, Buss PE, Helden PDv, Miller MA, Parsons SD. Development of gene expression assays measuring immune responses in the spotted hyena (Crocuta crocuta). African Zoology 2017;52:99–104. doi: doi: 10.1080/15627020.2017.1309300. [DOI] [Google Scholar]
- 61.Cystic fibrosis foundation patient registry 2020 annual data report. Bethesda, MD. [Google Scholar]
- 62.Hall-Stoodley L, McCoy KS. Biofilm aggregates and the host airway-microbial interface. Front Cell Infect Microbiol 2022;12:969326. doi: 10.3389/fcimb.2022.969326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bruscia EM, Bonfield TL. Cystic Fibrosis Lung Immunity: The Role of the Macrophage. J Innate Immun 2016;8:550–563. doi: 10.1159/000446825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Clary G, Sasindran SJ, Nesbitt N, Mason L, Cole S, Azad A, McCoy K, Schlesinger LS, Hall-Stoodley L. Mycobacterium abscessus Smooth and Rough Morphotypes Form Antimicrobial-Tolerant Biofilm Phenotypes but Are Killed by Acetic Acid. Antimicrob Agents Chemother 2018;62 doi: 10.1128/AAC.01782-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hall-Stoodley L Macrophages from CF Patients Are Susceptible to Rough Mycobacterium Abscessus;\. Pediatric pulmonology; Wiley: Hoboken, NJ, USA: 2019:S268. [Google Scholar]
- 66.Ayyappan JP, Ganapathi U, Lizardo K, Vinnard C, Subbian S, Perlin DS, Nagajyothi JF. Adipose Tissue Regulates Pulmonary Pathology during TB Infection. mBio 2019;10 doi: 10.1128/mBio.02771-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oswal N, Lizardo K, Dhanyalayam D, Ayyappan JP, Thangavel H, Heysell SK, Nagajyothi JF. Host Metabolic Changes during Mycobacterium Tuberculosis Infection Cause Insulin Resistance in Adult Mice. J Clin Med 2022;11 doi: 10.3390/jcm11061646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cadena J, Rathinavelu S, Lopez-Alvarenga JC, Restrepo BI. The re-emerging association between tuberculosis and diabetes: Lessons from past centuries. Tuberculosis (Edinb) 2019;116S:S89–S97. doi: 10.1016/j.tube.2019.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Semba RD, Darnton-Hill I, de Pee S. Addressing tuberculosis in the context of malnutrition and HIV coinfection. Food Nutr Bull 2010;31:S345–364. [PubMed] [Google Scholar]
- 70.Podell BK, Aibana O, Huang CC, DiLisio JE, Harris MC, Ackart DF, Armann K, Grover A, Severe P, Juste MAJ, Dupnik K, Basaraba RJ, Murray MB. The Impact of Vitamin A Deficiency on Tuberculosis Progression. Clin Infect Dis 2022;75:2178–2185. doi: 10.1093/cid/ciac326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aibana O, Franke MF, Huang CC, Galea JT, Calderon R, Zhang Z, Becerra MC, Smith ER, Ronnenberg AG, Contreras C, Yataco R, Lecca L, Murray MB. Impact of Vitamin A and Carotenoids on the Risk of Tuberculosis Progression. Clin Infect Dis 2017;65:900–909. doi: 10.1093/cid/cix476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Podell BK, Ackart DF, Obregon-Henao A, Eck SP, Henao-Tamayo M, Richardson M, Orme IM, Ordway DJ, Basaraba RJ. Increased severity of tuberculosis in Guinea pigs with type 2 diabetes: a model of diabetes-tuberculosis comorbidity. Am J Pathol 2014;184:1104–1118. doi: 10.1016/j.ajpath.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vallerskog T, Martens GW, Kornfeld H. Diabetic mice display a delayed adaptive immune response to Mycobacterium tuberculosis. J Immunol 2010;184:6275–6282. doi: 10.4049/jimmunol.1000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Martens GW, Arikan MC, Lee J, Ren F, Greiner D, Kornfeld H. Tuberculosis susceptibility of diabetic mice. Am J Respir Cell Mol Biol 2007;37:518–524. doi: 10.1165/rcmb.2006-0478OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Podell BK, Ackart DF, Kirk NM, Eck SP, Bell C, Basaraba RJ. Non-diabetic hyperglycemia exacerbates disease severity in Mycobacterium tuberculosis infected guinea pigs. PLoS One 2012;7:e46824. doi: 10.1371/journal.pone.0046824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kiran D, Podell BK, Chambers M, Basaraba RJ. Host-directed therapy targeting the Mycobacterium tuberculosis granuloma: a review. Semin Immunopathol 2016;38:167–183. doi: 10.1007/s00281-015-0537-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jones MM, Winthrop KL, Nelson SD, Duvall SL, Patterson OV, Nechodom KE, Findley KE, Radonovich LJ Jr., Samore MH, Fennelly KP. Epidemiology of nontuberculous mycobacterial infections in the U.S. Veterans Health Administration. PLoS One 2018;13:e0197976. doi: 10.1371/journal.pone.0197976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fennelly KP, Ojano-Dirain C, Yang Q, Liu L, Lu L, Progulske-Fox A, Wang GP, Antonelli P, Schultz G. Biofilm Formation by Mycobacterium abscessus in a Lung Cavity. Am J Respir Crit Care Med 2016;193:692–693. doi: 10.1164/rccm.201508-1586IM. [DOI] [PubMed] [Google Scholar]
- 79.Gupta T, Somanna N, Rowe T, LaGatta M, Helms S, Owino SO, Jelesijevic T, Harvey S, Jacobs W, Voss T, Sakamoto K, Day C, Whalen C, Karls R, He B, Tompkins SM, Bakre A, Ross T, Quinn FD. Ferrets as a model for tuberculosis transmission. Front Cell Infect Microbiol 2022;12:873416. doi: 10.3389/fcimb.2022.873416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mehaffy C, Ryan JM, Kruh-Garcia NA, Dobos KM. Extracellular Vesicles in Mycobacteria and Tuberculosis. Front Cell Infect Microbiol 2022;12:912831. doi: 10.3389/fcimb.2022.912831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Naqvi KF, Huante MB, Saito TB, Endsley MA, Gelman BB, Endsley JJ. Novel Role for Macrophage Galactose-Type Lectin-1 to Regulate Innate Immunity against Mycobacterium tuberculosis. J Immunol 2021;207:221–233. doi: 10.4049/jimmunol.2001276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Naqvi KF, Endsley JJ. Myeloid C-Type Lectin Receptors in Tuberculosis and HIV Immunity: Insights Into Co-infection? Front Cell Infect Microbiol 2020;10:263. doi: 10.3389/fcimb.2020.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Subbian S, Karakousis PC, Kaplan G. Rabbit model of mycobacterial diseases. Ch22 In Tuberculosis, Leprosy and Mycobacterial Diseases of Man and Animals; The Many Hosts of Mycobacteria Mukundan H, Chambers MA, Waters WR, Larsen MH (Eds) Cabi Publications, Boston, MA, USA: 2015:402–418. doi: 10.1079/9781780643960.0402 [DOI] [Google Scholar]
- 84.Tsenova L, Fallows D, Kolloli A, Singh P, O’Brien P, Kushner N, Kaplan G, Subbian S. Inoculum size and traits of the infecting clinical strain define the protection level against Mycobacterium tuberculosis infection in a rabbit model. Eur J Immunol 2020;50:858–872. doi: 10.1002/eji.201948448. [DOI] [PubMed] [Google Scholar]
- 85.Vijayan KKV, Cross KA, Curtis AD 2nd, Van Rompay KKA, Pollara J, Fox CB, Tomai M, Hanke T, Fouda G, Hudgens MG, Permar SR, De Paris K. Early Post-Vaccination Gene Signatures Correlate With the Magnitude and Function of Vaccine-Induced HIV Envelope-Specific Plasma Antibodies in Infant Rhesus Macaques. Front Immunol 2022;13:840976. doi: 10.3389/fimmu.2022.840976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hagge DA, Parajuli P, Kunwar CB, Rana D, Thapa R, Neupane KD, Nicholls P, Adams LB, Geluk A, Shah M, Napit IB. Opening a Can of Worms: Leprosy Reactions and Complicit Soil-Transmitted Helminths. EBioMedicine 2017;23:119–124. doi: 10.1016/j.ebiom.2017.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Endsley JJ, Huante MB, Naqvi KF, Gelman BB, Endsley MA. Advancing our understanding of HIV co-infections and neurological disease using the humanized mouse. Retrovirology 2021;18:14. doi: 10.1186/s12977-021-00559-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huante MB, Saito TB, Nusbaum RJ, Naqvi KF, Chauhan S, Hunter RL, Actor JK, Rudra JS, Endsley MA, Lisinicchia JG, Gelman BB, Endsley JJ. Small Animal Model of Post-chemotherapy Tuberculosis Relapse in the Setting of HIV Co-infection. Front Cell Infect Microbiol 2020;10:150. doi: 10.3389/fcimb.2020.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nusbaum RJ, Calderon VE, Huante MB, Sutjita P, Vijayakumar S, Lancaster KL, Hunter RL, Actor JK, Cirillo JD, Aronson J, Gelman BB, Lisinicchia JG, Valbuena G, Endsley JJ. Pulmonary Tuberculosis in Humanized Mice Infected with HIV-1. Sci Rep 2016;6:21522. doi: 10.1038/srep21522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fan X, Xu J, Files M, Cirillo JD, Endsley JJ, Zhou J, Endsley MA. Dual activity of niclosamide to suppress replication of integrated HIV-1 and Mycobacterium tuberculosis (Beijing). Tuberculosis (Edinb) 2019;116S:S28–S33. doi: 10.1016/j.tube.2019.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Simonson AW, Umstead TM, Lawanprasert A, Klein B, Almarzooqi S, Halstead ES, Medina SH. Extracellular matrix-inspired inhalable aerogels for rapid clearance of pulmonary tuberculosis. Biomaterials 2021;273:120848. doi: 10.1016/j.biomaterials.2021.120848. [DOI] [PubMed] [Google Scholar]
- 92.Darrah PA, Zeppa JJ, Maiello P, Hackney JA, Wadsworth MH 2nd, Hughes TK, Pokkali S, Swanson PA 2nd, Grant NL, Rodgers MA, Kamath M, Causgrove CM, Laddy DJ, Bonavia A, Casimiro D, Lin PL, Klein E, White AG, Scanga CA, Shalek AK, Roederer M, Flynn JL, Seder RA. Prevention of tuberculosis in macaques after intravenous BCG immunization. Nature 2020;577:95–102. doi: 10.1038/s41586-019-1817-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hassell JE, Baratta MV, Fallon IP, Siebler PH, Karns BL, Nguyen KT, Gates CA, Fonken LK, Frank MG, Maier SF, Lowry CA. Immunization with a heat-killed preparation of Mycobacterium vaccae NCTC 11659 enhances auditory-cued fear extinction in a stress-dependent manner. Brain Behav Immun 2023;107:1–15. doi: 10.1016/j.bbi.2022.09.003. [DOI] [PubMed] [Google Scholar]
- 94.Foxx CL, Heinze JD, Gonzalez A, Vargas F, Baratta MV, Elsayed AI, Stewart JR, Loupy KM, Arnold MR, Flux MC, Sago SA, Siebler PH, Milton LN, Lieb MW, Hassell JE, Smith DG, Lee KAK, Appiah SA, Schaefer EJ, Panitchpakdi M, Sikora NC, Weldon KC, Stamper CE, Schmidt D, Duggan DA, Mengesha YM, Ogbaselassie M, Nguyen KT, Gates CA, Schnabel K, Tran L, Jones JD, Vitaterna MH, Turek FW, Fleshner M, Dorrestein PC, Knight R, Wright KP, Lowry CA. Effects of Immunization With the Soil-Derived Bacterium Mycobacterium vaccae on Stress Coping Behaviors and Cognitive Performance in a “Two Hit” Stressor Model. Front Physiol 2020;11:524833. doi: 10.3389/fphys.2020.524833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Leard LE, Holm AM, Valapour M, Glanville AR, Attawar S, Aversa M, Campos SV, Christon LM, Cypel M, Dellgren G, Hartwig MG, Kapnadak SG, Kolaitis NA, Kotloff RM, Patterson CM, Shlobin OA, Smith PJ, Sole A, Solomon M, Weill D, Wijsenbeek MS, Willemse BWM, Arcasoy SM, Ramos KJ. Consensus document for the selection of lung transplant candidates: An update from the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2021;40:1349–1379. doi: 10.1016/j.healun.2021.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lobo LJ, Chang LC, Esther CR Jr., Gilligan PH, Tulu Z, Noone PG. Lung transplant outcomes in cystic fibrosis patients with pre-operative Mycobacterium abscessus respiratory infections. Clin Transplant 2013;27:523–529. doi: 10.1111/ctr.12140. [DOI] [PubMed] [Google Scholar]
- 97.Kavaliunaite E, Harris KA, Aurora P, Dixon G, Shingadia D, Muthialu N, Spencer H. Outcome according to subspecies following lung transplantation in cystic fibrosis pediatric patients infected with Mycobacterium abscessus. Transpl Infect Dis 2020;22:e13274. doi: 10.1111/tid.13274. [DOI] [PubMed] [Google Scholar]
- 98.Marty PK, Yetmar ZA, Gerberi DJ, Escalante P, Pennington KM, Mahmood M. Risk factors and outcomes of non-tuberculous mycobacteria infection in lung transplant recipients: A systematic review and meta-analysis. J Heart Lung Transplant 2023;42:264–274. doi: 10.1016/j.healun.2022.10.004. [DOI] [PubMed] [Google Scholar]
- 99.Dedrick RM, Smith BE, Cristinziano M, Freeman KG, Jacobs-Sera D, Belessis Y, Whitney Brown A, Cohen KA, Davidson RM, van Duin D, Gainey A, Garcia CB, Robert George CR, Haidar G, Ip W, Iredell J, Khatami A, Little JS, Malmivaara K, McMullan BJ, Michalik DE, Moscatelli A, Nick JA, Tupayachi Ortiz MG, Polenakovik HM, Robinson PD, Skurnik M, Solomon DA, Soothill J, Spencer H, Wark P, Worth A, Schooley RT, Benson CA, Hatfull GF. Phage Therapy of Mycobacterium Infections: Compassionate Use of Phages in 20 Patients With Drug-Resistant Mycobacterial Disease. Clin Infect Dis 2023;76:103–112. doi: 10.1093/cid/ciac453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hayes D Jr., Shukla RK, Cheng Y, Gecili E, Merling MR, Szczesniak RD, Ziady AG, Woods JC, Hall-Stoodley L, Liyanage NP, Robinson RT. Tissue-localized immune responses in people with cystic fibrosis and respiratory nontuberculous mycobacteria infection. JCI Insight 2022;7 doi: 10.1172/jci.insight.157865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mercaldo RA, Marshall JE, Prevots DR, Lipner EM, French JP. Detecting clusters of high nontuberculous mycobacteria infection risk for persons with cystic fibrosis – An analysis of U.S. counties. Tuberculosis (Edinb) 2023;138:102296. doi: 10.1016/j.tube.2022.102296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Blakney RA, Ricotta EE, Frankland TB, Honda S, Zelazny A, Mayer-Barber KD, Dean SG, Follmann D, Olivier KN, Daida YG, Prevots DR. Incidence of Nontuberculous Mycobacterial Pulmonary Infection, by Ethnic Group, Hawaii, USA, 2005-2019. Emerg Infect Dis 2022;28:1543–1550. doi: 10.3201/eid2808.212375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dean SG, Ricotta EE, Fintzi J, Lai YL, Kadri SS, Olivier KN, Zelazny A, Prevots DR. Mycobacterial Testing Trends, United States, 2009-2015(1). Emerg Infect Dis 2020;26:2243–2246. doi: 10.3201/eid2609.200749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Winthrop KL, Marras TK, Adjemian J, Zhang H, Wang P, Zhang Q. Incidence and Prevalence of Nontuberculous Mycobacterial Lung Disease in a Large U.S. Managed Care Health Plan, 2008-2015. Ann Am Thorac Soc 2020;17:178–185. doi: 10.1513/AnnalsATS.201804-236OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Adjemian J, Daniel-Wayman S, Ricotta E, Prevots DR. Epidemiology of Nontuberculous Mycobacteriosis. Semin Respir Crit Care Med 2018;39:325–335. doi: 10.1055/s-0038-1651491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Thomson RM, Armstrong JG, Looke DF. Gastroesophageal reflux disease, acid suppression, and Mycobacterium avium complex pulmonary disease. Chest 2007;131:1166–1172. doi: 10.1378/chest.06-1906. [DOI] [PubMed] [Google Scholar]
- 107.Saeed DK, Shakoor S, Irfan S, Hasan R. Mycobacterial contamination of bronchoscopes: Challenges and possible solutions in low resource settings. Int J Mycobacteriol 2016;5:408–411. doi: 10.1016/j.ijmyco.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 108.Bouso JM, Burns JJ, Amin R, Livingston FR, Elidemir O. Household proximity to water and nontuberculous mycobacteria in children with cystic fibrosis. Pediatr Pulmonol 2017;52:324–330. doi: 10.1002/ppul.23646. [DOI] [PubMed] [Google Scholar]
- 109.Falkinham JO 3rd. Environmental sources of nontuberculous mycobacteria. Clin Chest Med 2015;36:35–41. doi: 10.1016/j.ccm.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 110.Stanish LF, Hull NM, Robertson CE, Harris JK, Stevens MJ, Spear JR, Pace NR. Factors Influencing Bacterial Diversity and Community Composition in Municipal Drinking Waters in the Ohio River Basin, USA. PLoS One 2016;11:e0157966. doi: 10.1371/journal.pone.0157966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Falkinham JO, Pruden A, Edwards M. Opportunistic Premise Plumbing Pathogens: Increasingly Important Pathogens in Drinking Water. Pathogens 2015;4:373–386. doi: 10.3390/pathogens4020373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hull NM, Ling F, Pinto AJ, Albertsen M, Jang HG, Hong PY, Konstantinidis KT, LeChevallier M, Colwell RR, Liu WT. Drinking Water Microbiome Project: Is it Time? Trends Microbiol 2019;27:670–677. doi: 10.1016/j.tim.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 113.Song K, Mohseni M, Taghipour F. Application of ultraviolet light-emitting diodes (UV-LEDs) for water disinfection: A review. Water Res 2016;94:341–349. doi: 10.1016/j.watres.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 114.Hull NM, Herold WH, Linden KG. UV LED Water Disinfection: Validation and Small System Demonstration Study. American Water Works Association Water Science 2019;e1148 doi: doi: 10.1002/aws2.1148. [DOI] [Google Scholar]
- 115.Hayes SL, Sivaganesan M, White KM, Pfaller SL. Assessing the effectiveness of low-pressure ultraviolet light for inactivating Mycobacterium avium complex (MAC) micro-organisms. Lett Appl Microbiol 2008;47:386–392. doi: 10.1111/j.1472-765X.2008.02442.x. [DOI] [PubMed] [Google Scholar]
- 116.Beck SE, Wright HB, Hargy TM, Larason TC, Linden KG. Action spectra for validation of pathogen disinfection in medium-pressure ultraviolet (UV) systems. Water Res 2015;70:27–37. doi: 10.1016/j.watres.2014.11.028. [DOI] [PubMed] [Google Scholar]
- 117.Beck SE, Rodriguez RA, Linden KG, Hargy TM, Larason TC, Wright HB. Wavelength dependent UV inactivation and DNA damage of adenovirus as measured by cell culture infectivity and long range quantitative PCR. Environ Sci Technol 2014;48:591–598. doi: 10.1021/es403850b. [DOI] [PubMed] [Google Scholar]
- 118.Harm W Biological Effects of Ultraviolet Radiation. Camgridge University Press: Cambridge, 1980. [Google Scholar]
- 119.Rook GA, Adams V, Hunt J, Palmer R, Martinelli R, Brunet LR. Mycobacteria and other environmental organisms as immunomodulators for immunoregulatory disorders. Springer Semin Immunopathol 2004;25:237–255. doi: 10.1007/s00281-003-0148-9. [DOI] [PubMed] [Google Scholar]
- 120.Rook GA, Lowry CA. The hygiene hypothesis and psychiatric disorders. Trends Immunol 2008;29:150–158. doi: 10.1016/j.it.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 121.Sonnenburg ED, Sonnenburg JL. Starving our microbial self: the deleterious consequences of a diet deficient in microbiota-accessible carbohydrates. Cell Metab 2014;20:779–786. doi: 10.1016/j.cmet.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sonnenburg JL, Sonnenburg ED. Vulnerability of the industrialized microbiota. Science 2019;366 doi: 10.1126/science.aaw9255. [DOI] [PubMed] [Google Scholar]
- 123.Atherton JC, Blaser MJ. Coadaptation of Helicobacter pylori and humans: ancient history, modern implications. J Clin Invest 2009;119:2475–2487. doi: 10.1172/JCI38605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lowry CA, Smith DG, Siebler PH, Schmidt D, Stamper CE, Hassell JE Jr., Yamashita PS, Fox JH, Reber SO, Brenner LA, Hoisington AJ, Postolache TT, Kinney KA, Marciani D, Hernandez M, Hemmings SM, Malan-Muller S, Wright KP, Knight R, Raison CL, Rook GA. The Microbiota, Immunoregulation, and Mental Health: Implications for Public Health. Curr Environ Health Rep 2016;3:270–286. doi: 10.1007/s40572-016-0100-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rook GA, Raison CL, Lowry CA. Microbial ‘old friends’, immunoregulation and socioeconomic status. Clin Exp Immunol 2014;177:1–12. doi: 10.1111/cei.12269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ohnmacht C, Park JH, Cording S, Wing JB, Atarashi K, Obata Y, Gaboriau-Routhiau V, Marques R, Dulauroy S, Fedoseeva M, Busslinger M, Cerf-Bensussan N, Boneca IG, Voehringer D, Hase K, Honda K, Sakaguchi S, Eberl G. MUCOSAL IMMUNOLOGY. The microbiota regulates type 2 immunity through RORgammat(+) T cells. Science 2015;349:989–993. doi: 10.1126/science.aac4263. [DOI] [PubMed] [Google Scholar]
- 127.Sefik E, Geva-Zatorsky N, Oh S, Konnikova L, Zemmour D, McGuire AM, Burzyn D, Ortiz-Lopez A, Lobera M, Yang J, Ghosh S, Earl A, Snapper SB, Jupp R, Kasper D, Mathis D, Benoist C. MUCOSAL IMMUNOLOGY. Individual intestinal symbionts induce a distinct population of RORgamma(+) regulatory T cells. Science 2015;349:993–997. doi: 10.1126/science.aaa9420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Langgartner D, Lowry CA, Reber SO. Old Friends, immunoregulation, and stress resilience. Pflugers Arch 2019;471:237–269. doi: 10.1007/s00424-018-2228-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Amoroso M, Langgartner D, Lowry CA, Reber SO. Rapidly Growing Mycobacterium Species: The Long and Winding Road from Tuberculosis Vaccines to Potent Stress-Resilience Agents. Int J Mol Sci 2021;22 doi: 10.3390/ijms222312938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Macovei L, McCafferty J, Chen T, Teles F, Hasturk H, Paster BJ, Campos-Neto A. The hidden ‘mycobacteriome’ of the human healthy oral cavity and upper respiratory tract. J Oral Microbiol 2015;7:26094. doi: 10.3402/jom.v7.26094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hansen GA. On the Etiology of Leprosy. Br Foreign Med Chir Rev 1875;55:459–489. [PMC free article] [PubMed] [Google Scholar]
- 132.Hansen GA. Undersogelser angraaende spedalskhedens aasager. Nordsk Magazin for Laegervidenskaben (Supplement) 1874:1–88. [Google Scholar]
- 133.Irgens LM. Leprosy in Norway. An epidemiological study based on a national patient registry. Lepr Rev 1980;51 Suppl 1:i–xi, 1-130. [PubMed] [Google Scholar]
- 134.Meima A, Irgens LM, van Oortmarssen GJ, Richardus JH, Habbema JD. Disappearance of leprosy from Norway: an exploration of critical factors using an epidemiological modelling approach. Int J Epidemiol 2002;31:991–1000. doi: 10.1093/ije/31.5.991. [DOI] [PubMed] [Google Scholar]
- 135.Irgens LM. Secular trends in leprosy: increase in age at onset associated with declining rates and long incubation periods. Int J Lepr Other Mycobact Dis 1985;53:610–617. [PubMed] [Google Scholar]
- 136.WHO. Global leprosy (Hansen disease) update, 2019: time to step-up prevention initiatives. Wkly Epid Rec 2020;95:417–440. [Google Scholar]
- 137.Barreto JG, Frade MAC, Bernardes Filho F, da Silva MB, Spencer JS, Salgado CG. Leprosy in Children. Curr Infect Dis Rep 2017;19:23. doi: 10.1007/s11908-017-0577-6. [DOI] [PubMed] [Google Scholar]
- 138.Kerr-Pontes LR, Barreto ML, Evangelista CM, Rodrigues LC, Heukelbach J, Feldmeier H. Socioeconomic, environmental, and behavioural risk factors for leprosy in North-east Brazil: results of a case-control study. Int J Epidemiol 2006;35:994–1000. doi: 10.1093/ije/dyl072. [DOI] [PubMed] [Google Scholar]
- 139.Pescarini JM, Strina A, Nery JS, Skalinski LM, Andrade KVF, Penna MLF, Brickley EB, Rodrigues LC, Barreto ML, Penna GO. Socioeconomic risk markers of leprosy in high-burden countries: A systematic review and meta-analysis. PLoS Negl Trop Dis 2018;12:e0006622. doi: 10.1371/journal.pntd.0006622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.da Silva MB, Li W, Bouth RC, Gobbo AR, Messias ACC, Moraes TMP, Jorge EVO, Barreto JG, Filho FB, Conde GAB, Frade MAC, Salgado CG, Spencer JS. Latent leprosy infection identified by dual RLEP and anti-PGL-I positivity: Implications for new control strategies. PLoS One 2021;16:e0251631. doi: 10.1371/journal.pone.0251631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nobre ML, Illarramendi X, Dupnik KM, Hacker MA, Nery JA, Jeronimo SM, Sarno EN. Multibacillary leprosy by population groups in Brazil: Lessons from an observational study. PLoS Negl Trop Dis 2017;11:e0005364. doi: 10.1371/journal.pntd.0005364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nazario AP, Ferreira J, Schuler-Faccini L, Fiegenbaum M, Artigalas O, Vianna FSL. Leprosy in Southern Brazil: a twenty-year epidemiological profile. Rev Soc Bras Med Trop 2017;50:251–255. doi: 10.1590/0037-8682-0229-2016. [DOI] [PubMed] [Google Scholar]
- 143.Collins JH, Lenz SM, Ray NA, Balagon MF, Hagge DA, Lahiri R, Adams LB. A Sensitive and Quantitative Assay to Enumerate and Measure Mycobacterium leprae Viability in Clinical and Experimental Specimens. Curr Protoc 2022;2:e359. doi: 10.1002/cpz1.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Davis GL, Ray NA, Lahiri R, Gillis TP, Krahenbuhl JL, Williams DL, Adams LB. Molecular assays for determining Mycobacterium leprae viability in tissues of experimentally infected mice. PLoS Negl Trop Dis 2013;7:e2404. doi: 10.1371/journal.pntd.0002404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bailey MA, Na H, Duthie MS, Gillis TP, Lahiri R, Parish T. Nitazoxanide is active against Mycobacterium leprae. PLoS One 2017;12:e0184107. doi: 10.1371/journal.pone.0184107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Franco-Paredes C, Montes de Oca Sanchez G, White C. Global Leprosy Status in 2020: Still Losing Touch. Ann Acad Med Singap 2020;49:1–2. [PubMed] [Google Scholar]
- 147.White C, Franco-Paredes C. Leprosy in the 21st century. Clin Microbiol Rev 2015;28:80–94. doi: 10.1128/CMR.00079-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Franco-Paredes C, Santos-Preciado JI. Freedom, justice, and neglected tropical diseases. PLoS Negl Trop Dis 2011;5:e1235. doi: 10.1371/journal.pntd.0001235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pena MT, Lahiri R, Ebenezer GJ, Wheat SW, Figarola J, Truman RW, Adams LB. The Armadillo as a Model for Leprosy Nerve Function Impairment: Preventative and Therapeutic Interventions. Front Med (Lausanne) 2022;9:879097. doi: 10.3389/fmed.2022.879097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Duthie MS, Pena MT, Ebenezer GJ, Gillis TP, Sharma R, Cunningham K, Polydefkis M, Maeda Y, Makino M, Truman RW, Reed SG. LepVax, a defined subunit vaccine that provides effective pre-exposure and post-exposure prophylaxis of M. leprae infection. NPJ Vaccines 2018;3:12. doi: 10.1038/s41541-018-0050-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lustosa AA, Nogueira LT, Pedrosa JI, Teles JB, Campelo V. The impact of leprosy on health-related quality of life. Rev Soc Bras Med Trop 2011;44:621–626. doi: 10.1590/s0037-86822011000500019. [DOI] [PubMed] [Google Scholar]
- 152.Dupnik KM, Bair TB, Maia AO, Amorim FM, Costa MR, Keesen TS, Valverde JG, Queiroz Mdo C, Medeiros LL, de Lucena NL, Wilson ME, Nobre ML, Johnson WD Jr., Jeronimo SM. Transcriptional changes that characterize the immune reactions of leprosy. J Infect Dis 2015;211:1658–1676. doi: 10.1093/infdis/jiu612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Legall N, Salvador LCM. Selective sweep sites and SNP dense regions differentiate Mycobacterium bovis isolates across scales. Front Microbiol 2022;13:787856. doi: 10.3389/fmicb.2022.787856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Crispell J, Benton CH, Balaz D, De Maio N, Ahkmetova A, Allen A, Biek R, Presho EL, Dale J, Hewinson G, Lycett SJ, Nunez-Garcia J, Skuce RA, Trewby H, Wilson DJ, Zadoks RN, Delahay RJ, Kao RR. Combining genomics and epidemiology to analyse bi-directional transmission of Mycobacterium bovis in a multi-host system. Elife 2019;8 doi: 10.7554/eLife.45833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Crispell J, Zadoks RN, Harris SR, Paterson B, Collins DM, de-Lisle GW, Livingstone P, Neill MA, Biek R, Lycett SJ, Kao RR, Price-Carter M. Using whole genome sequencing to investigate transmission in a multi-host system: bovine tuberculosis in New Zealand. BMC Genomics 2017;18:180. doi: 10.1186/s12864-017-3569-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Salvador LCM, O’Brien DJ, Cosgrove MK, Stuber TP, Schooley AM, Crispell J, Church SV, Grohn YT, Robbe-Austerman S, Kao RR. Disease management at the wildlife-livestock interface: Using whole-genome sequencing to study the role of elk in Mycobacterium bovis transmission in Michigan, USA. Mol Ecol 2019;28:2192–2205. doi: 10.1111/mec.15061. [DOI] [PubMed] [Google Scholar]
- 157.Gormley E, Ni Bhuachalla D, Fitzsimons T, O’Keeffe J, McGrath G, Madden JM, Fogarty N, Kenny K, Messam LLM, Murphy D, Corner LAL. Protective immunity against tuberculosis in a free-living badger population vaccinated orally with Mycobacterium bovis Bacille Calmette-Guerin. Transbound Emerg Dis 2022;69:e10–e19. doi: 10.1111/tbed.14254. [DOI] [PubMed] [Google Scholar]
- 158.Lesellier S, Birch CPD, Dave D, Dalley D, Gowtage S, Palmer S, McKenna C, Williams GA, Ashford R, Weyer U, Beatham S, Coats J, Nunez A, Sanchez-Cordon P, Spiropoulos J, Powell S, Sawyer J, Pascoe J, Hendon-Dunn C, Bacon J, Chambers MA. Bioreactor-Grown Bacillus of Calmette and Guerin (BCG) Vaccine Protects Badgers against Virulent Mycobacterium bovis When Administered Orally: Identifying Limitations in Baited Vaccine Delivery. Pharmaceutics 2020;12 doi: 10.3390/pharmaceutics12080782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lesellier S, Boschiroli ML, Barrat J, Wanke C, Salguero FJ, Garcia-Jimenez WL, Nunez A, Godinho A, Spiropoulos J, Palmer S, Dave D, Anderson P, Boucher JM, de Cruz K, Henault S, Michelet L, Gowtage S, Williams GA, Nadian AK, Monchatre-Leroy E, Boue F, Chambers MA, Richomme C. Detection of live M. bovis BCG in tissues and IFN-gamma responses in European badgers (Meles meles) vaccinated by oropharyngeal instillation or directly in the ileum. BMC Vet Res 2019;15:445. doi: 10.1186/s12917-019-2166-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Balseiro A, Merediz I, Sevilla IA, Garcia-Castro C, Gortazar C, Prieto JM, Delahay RJ. Infection of Eurasian badgers (Meles meles) with Mycobacterium avium complex (MAC) bacteria. Vet J 2011;188:231–233. doi: 10.1016/j.tvjl.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 161.Birch CPD, Chambers MA, Lesellier S. A combined measure of tuberculous lesions for assessing the efficacy of vaccination against tuberculosis (Mycobacterium bovis) in European badgers (Meles meles) supports the 3Rs principle of reduction. Vaccine 2021;39:1661–1666. doi: 10.1016/j.vaccine.2019.10.079. [DOI] [PubMed] [Google Scholar]
- 162.Lesellier S Immunological responses of European badgers (Meles Meles) to infection with Mycobacterium bovis. Comp Immunol Microbiol Infect Dis 2018;61:9–15. doi: 10.1016/j.cimid.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 163.Bhattacharya B, Xiao S, Chatterjee S, Urbanowski M, Ordonez A, Ihms EA, Agrahari G, Lun S, Berland R, Pichugin A, Gao Y, Connor J, Ivanov AR, Yan BS, Kobzik L, Koo BB, Jain S, Bishai W, Kramnik I. The integrated stress response mediates necrosis in murine Mycobacterium tuberculosis granulomas. J Clin Invest 2021;131 doi: 10.1172/JCI130319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tavolara TE, Niazi MKK, Ginese M, Piedra-Mora C, Gatti DM, Beamer G, Gurcan MN. Automatic discovery of clinically interpretable imaging biomarkers for Mycobacterium tuberculosis supersusceptibility using deep learning. EBioMedicine 2020;62:103094. doi: 10.1016/j.ebiom.2020.103094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kus P, Gurcan MN, Beamer G. Automatic Detection of Granuloma Necrosis in Pulmonary Tuberculosis Using a Two-Phase Algorithm: 2D-TB. Microorganisms 2019;7 doi: 10.3390/microorganisms7120661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Judd JA, Canestrari J, Clark R, Joseph A, Lapierre P, Lasek-Nesselquist E, Mir M, Palumbo M, Smith C, Stone M, Upadhyay A, Wirth SE, Dedrick RM, Meier CG, Russell DA, Dills a, Dove E, Kester J, Wolf ID, Zhu J, Rubin ER, Fortune S, Hatfull GF, Gray TA, Wade JT, Derbyshire KM. A Mycobacterial Systems Resource for the Research Community. mBio 2021;12 doi: 10.1128/mBio.02401-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhu J, Wolf ID, Dulberger CL, Won HI, Kester JC, Judd JA, Wirth SE, Clark RR, Li Y, Luo Y, Gray TA, Wade JT, Derbyshire KM, Fortune SM, Rubin EJ. Spatiotemporal localization of proteins in mycobacteria. Cell Rep 2021;37:110154. doi: 10.1016/j.celrep.2021.110154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Paudel S, Brenner EP, Hadi SA, Suzuki Y, Nakajima C, Tsubota T, Gairhe KP, Maharjan B, Sreevatsan S. Genome Sequences of Two Mycobacterium tuberculosis Isolates from Asian Elephants in Nepal. Microbiol Resour Announc 2021;10:e0061421. doi: 10.1128/MRA.00614-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Cohen SB, Gern BH, Urdahl KB. The Tuberculous Granuloma and Preexisting Immunity. Annu Rev Immunol 2022;40:589–614. doi: 10.1146/annurev-immunol-093019-125148. [DOI] [PubMed] [Google Scholar]
- 170.Ordonez AA, Tucker EW, Anderson CJ, Carter CL, Ganatra S, Kaushal D, Kramnik I, Lin PL, Madigan CA, Mendez S, Rao J, Savic RM, Tobin DM, Walzl G, Wilkinson RJ, Lacourciere KA, Via LE, Jain SK. Visualizing the dynamics of tuberculosis pathology using molecular imaging. J Clin Invest 2021;131 doi: 10.1172/JCI145107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Carow B, Hauling T, Qian X, Kramnik I, Nilsson M, Rottenberg ME. Spatial and temporal localization of immune transcripts defines hallmarks and diversity in the tuberculosis granuloma. Nat Commun 2019;10:1823. doi: 10.1038/s41467-019-09816-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. https://msrdb.org .
- 173. https://www.wadsworth.org/research/scientific-resources/interactive-genomics .
- 174.Cronan MR, Hughes EJ, Brewer WJ, Viswanathan G, Hunt EG, Singh B, Mehra S, Oehlers SH, Gregory SG, Kaushal D, Tobin DM. A non-canonical type 2 immune response coordinates tuberculous granuloma formation and epithelialization. Cell 2021;184:1757–1774 e1714. doi: 10.1016/j.cell.2021.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Saelens JW, Sweeney MI, Viswanathan G, Xet-Mull AM, Jurcic Smith KL, Sisk DM, Hu DD, Cronin RM, Hughes EJ, Brewer WJ, Coers J, Champion MM, Champion PA, Lowe CB, Smith CM, Lee S, Stout JE, Tobin DM. An ancestral mycobacterial effector promotes dissemination of infection. Cell 2022;185:4507–4525 e4518. doi: 10.1016/j.cell.2022.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Larsen MH, Lacourciere K, Parker TM, Kraigsley A, Achkar JM, Adams LB, Dupnik KM, Hall-Stoodley L, Hartman T, Kanipe C, Kurtz SL, Miller MA, Salvador LCM, Spencer JS, Robinson RT. The Many Hosts of Mycobacteria 8 (MHM8): A conference report. Tuberculosis (Edinb) 2020;121:101914. doi: 10.1016/j.tube.2020.101914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. https://drsusancenter.org .
- 178.Rosenbloom R, Gavrish I, Tseng AE, Seidel K, Yabaji SM, Gertje HP, Huber BR, Kramnik I, Crossland NA. Progression and Dissemination of Pulmonary Mycobacterium Avium Infection in a Susceptible Immunocompetent Mouse Model. Int J Mol Sci 2022;23 doi: 10.3390/ijms23115999. [DOI] [PMC free article] [PubMed] [Google Scholar]


