Figure 2.
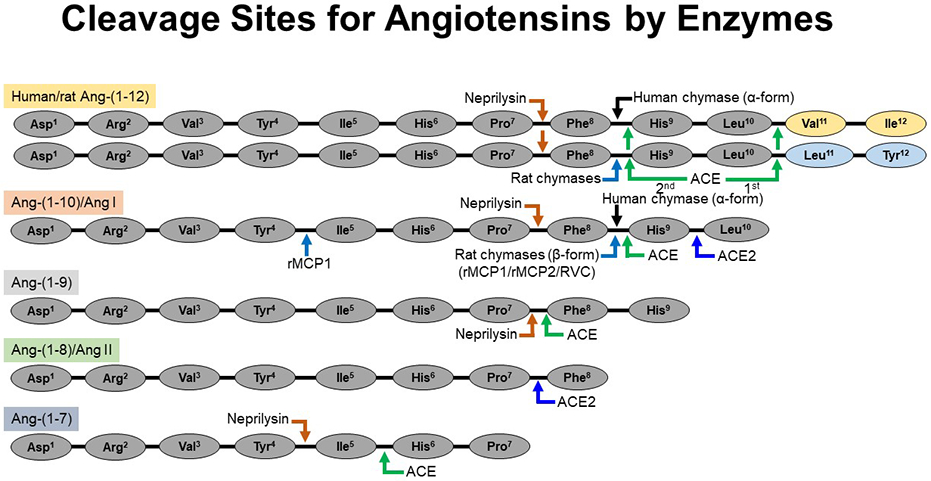
Cleavage sites for the processing of angiotensin peptides by chymase, ACE, ACE2, and neprilysin. Humans (α-chymase) and rat (β-chymases) directly cleave the Phe8-His9 bond of the Ang-(1-12) to generate Ang II directly. Similarly, rat β-chymases (rMCP-1, rMCP-2 and RVC) cleave the Phe8-His9 bond of the Ang I to generate Ang II directly. ACE cleaves Ang-(1-12) [sequentially, 1st Leu10-Val11 (human)/Leu10-Leu11 (rat) and then Phe8-His9 bonds] and Ang I [Phe8-His9 bond] to generate Ang II. ACE2 also cleaves the Ang I [Phe8-His9 bond] to generate Ang-(1-9). Ang-(1-12), Ang I and Ang-(1-9) are cleaved by neprilysin (an endopeptidase) at Pro7-Phe8 bond to generate Ang-(1-7). Rat β-chymase (rMCP-1) may hydrolyze Ang II at the Tyr4-Ile5 bond. Ang II is not further cleaved by human α-chymase but, ACE2 cleaves the Pro7-Phe8 bond of Ang II to generate Ang-(1-7). Ang-(1-7) is cleaved by ACE and neprilysin to generate Ang-(1-5) and Ang-(1-4), respectively. Abbreviations: rat mast cell protease-1, rMCP-1; rat mast cell protease-2, rMCP-2 and rat vascular chymase, RVC.
